To the Editor,
5-Fluorouracil (5-FU) has been the most active chemotherapeutic agent for metastatic colorectal cancer (CRC), and the mainstay of treatment for the last 40 years. The reported response rate to single-agent 5-FU ranges from 10–30%, yet the median survival remains approximately 12 months [Citation1]. Over the past decade, new agents oxaliplatin, irinotecan, Bevacizumab, Panitumumab, and Cetuximab, have been introduced into clinical practice. However, it is only when these agents are combined with 5-FU that significant improvements in response rates and median survival are seen [Citation1–5]. Consequently, development of 5-FU resistance in CRC patients presents a major challenge. Strategies to reverse cellular resistance to 5-FU may create a treatment choice for approximately 16,000 patients annually who otherwise have no alternative options. We recently demonstrated in vitro that arsenic trioxide (ATO) re-sensitized 5-FU resistant colorectal cancer cells [Citation6]. Investigation of the possible mode of action suggested ATO down-regulates the transcription of thymidylate synthase (TS). In a subsequent Phase I clinical trial, patients were administered 0.20 mg/kg ATO, 2,600 mg/m2 5-FU, and 500 mg/m2 leucovorin. This treatment regimen was well tolerated and chosen to be the recommended Phase II dose [Citation7].
Patients and methods
Following completion of our previous Phase I study, six patients with advanced, refractory mCRC were treated off protocol at the recommended Phase II dose [Citation7]. All patients have had histologically confirmed measurable stage IV CRC, which had been heavily pretreated and progressed through the following regimens: FOLFOX/ FOLFIRI with Bevacizumab, or Cetuximab with irinotecan. Three of the patients had k-ras wild type tumors, and had previously received three different chemotherapy regimens. The other three patients had k-ras mutated tumors, and had previously received two different treatments. Patients ranged in age from 50–70 years (median 65 years). Half of the patients were male (50%) and half female (50%), and the majority Hispanic (66.7%). Duration of treatment ranges from four to 12 months, with four patients still alive and two have expired. All patients, having progressed through the aforementioned regimens, were recognized to have 5-FU resistant tumors, requiring an alternative form of treatment.
Treatment scheme
A loading dose of ATO (0.20 mg/kg) was administered on days 1–5 by IV infusion over one to four hours to downregulate TS expression levels, and twice weekly thereafter to maintain TS downregulation. Following ATO administration, 5-FU and leucovorin were administered by IV infusion over 24 hours on days 8, 15, and 22. One treatment cycle spanned five weeks, which included four weeks of treatment followed by one week of rest [Citation7]. Imaging studies were conducted to assess radiological response after the completion of every two cycles of treatment (10 weeks).
Evaluation of toxicity
All patients were monitored for signs of cardiotoxicity (persistent hypertension, arrhythmias, and weight gain), neurotoxicity (lethargy and peripheral neuropathy), hematologic toxicity, hepatotoxicity, nephrotoxicity, and skin toxicity (). During cycle one, there were four episodes of grade 1 fatigue, one episode of grade 2 weight gain, three episodes of grade 1 diarrhea, two episodes of grade 1 neutropenia, one episode of grade 2 neutropenia, one episode of grade 1 peripheral edema, one episode of grade 2 peripheral edema, and one episode of grade 1 abdominal pain. In cycles 2–10, weight gain and nausea were the most common toxicities with six and five episodes, respectively. There were four total episodes each of fatigue, diarrhea, and peripheral edema. There was also one episode each of grade 1 and grade 2 abdominal pain, one episode each of grade 1 and grade 2 neutropenia, and one episode of grade 3 QTc prolongation.
Table I. Cycle 1 and cycles 2–10 treatment related toxicities by grade.
Clinical response and discussion
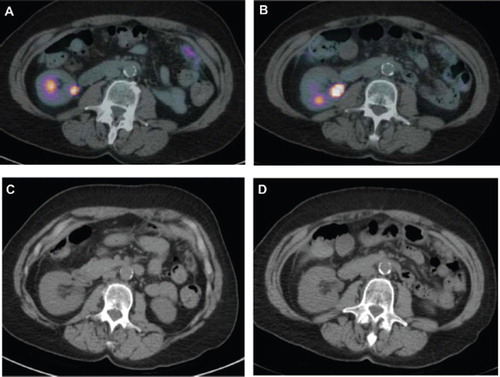
Clinical response was evaluated based on CT scans; however, other indices, like PET scans and routine CEA were also performed. Response and progression were assessed using the new international criteria proposed by the Response Evaluation Criteria in Solid Tumors Committee [Citation8]. Of the six patients, four have had stabilization of disease, and two have had partial responses ( and ). Stable disease is considered a positive response. As of May 2010, Patient 1 with partial response has been on treatment for ten cycles and is continuing to receive this regimen. Patient 2 with partial response received four cycles of treatment, and withdrew from receiving chemotherapy for personal reasons and subsequently expired at home. As seen in PET scans ( and ), Patient 1 showed a significant decrease in SUV uptake value. It decreased from a maximum of 9.28 in May 2009 to 2.12 in June 2010 (77% decrease). Additionally, CT scans showed a decrease in tumor size ( and ). Furthermore, CEA of Patient 1 decreased from 525 ng/ml at the beginning of treatment in May 2009 to 110 ng/ml in June 2010. Patient 2 presented with abdominal carcinomatosis and showed no bulky, however measurable disease. Patient 2 showed a decrease in SUV uptake from 4.03 in February 2010 to 2.51 in May 2010 (48% decrease) (). Patient 2 CEA dropped from 35 to 8 ng/ml. As of July 2010, two of four patients who had received four or five cycles of chemotherapy reported to have had stable disease. Both have progressed and are no longer receiving treatment. The third patient who had received four cycles has progressed and expired. The fourth patient is continuing with the treatment regimen and is receiving the seventh cycle of therapy.
Figure 1. Patient 1. PET scan from (A) May 2009 showing a maximum SUV uptake value of 9.28 and (B) June 2010 with a maximum SUV uptake value of 2.12. CT scan from (C) May 2009 and (D) June 2010.
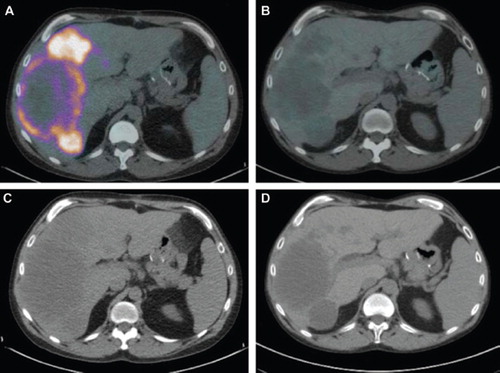
Figure 2. Patient 2. PET scans from (A) February 2010 showing a maximum SUV uptake value of 4.03 and (B) May 2010 with a maximum SUV uptake value of 2.51. CT scan from (C) February 2010 and (D) May 2010.
We and others have shown that one mechanism of 5-FU resistance in metastatic CRC patients is highly associated with gene amplification [Citation2] and overexpression of intratumoral TS mRNA [Citation9, Citation10]. We have shown ATO to inhibit TS at the mRNA level, effectively maximizing tumor response to 5-FU [Citation7]. Various studies have also found that ATO activates apoptosis in colon cancer cell lines through the Caspase-3 pathway and its substrate, PARP [Citation11,Citation12]. Additionally, arsenic trioxide may induce apoptosis by increased p21 protein expression, activating cell cycle arrest, as well as increased rapid expression of GADD proteins [Citation11]. Further research into the mechanisms of reversal of 5-FU resistance by ATO is warranted. The high response rate from this clinical experience (six of six patients) and manageable toxicity profile may indicate an effective alternative treatment option for 5-FU resistant patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Braun AH, Achterrath W, Wilke H, Vanhoefer U, Harstrick A, Preusser P. New systemic frontline treatment for metastatic colorectal carcinoma. Cancer 2004;100:1558–77.
- Wang TL, Diaz LA, Jr., Romans K, Bardelli A, Saha S, Galizia G, . Digital karyotyping identifies thymidylate synthase amplification as a mechanism of resistance to 5-fluorouracil in metastatic colorectal cancer patients. Proc Natl Acad Sci USA 2004;101:3089–94.
- Diaz-Rubio E, Sastre J, Zaniboni A, Labianca R, Cortes-Funes H, De Braud F, . Oxaliplatin as single agent in previously untreated colorectal carcinoma patients: A phase II multicentric study. Ann Oncol 1998;9:105–8.
- Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, . Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000;343:905–14.
- Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, . A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23–30.
- Subbarayan PR, Lee K, Ardalan B. Arsenic trioxide suppresses thymidylate synthase in 5-FU-resistant colorectal cancer cell line HT29 In Vitro re-sensitizing cells to 5-FU. Anticancer Res 2010;30:1157–62.
- Ardalan B, Subbarayan PR, Ramos Y, Gonzalez M, Fernandez A, Mezentsev D, . A phase I study of 5-fluorouracil/leucovorin and arsenic trioxide for patients with refractory/relapsed colorectal carcinoma. Clin Cancer Res 2010;16: 3019–27.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92: 205–16.
- Bathe OF, Franceschi D, Livingstone AS, Moffat FL, Tian E, Ardalan B. Increased thymidylate synthase gene expression in liver metastases from colorectal carcinoma: Implications for chemotherapeutic options and survival. Cancer J Sci Am 1999;5:34–40.
- Pare L, Marcuello E, Altes A, del Rio E, Sedano L, Barnadas A, . Transcription factor-binding sites in the thymidylate synthase gene: Predictors of outcome in patients with metastatic colorectal cancer treated with 5-fluorouracil and oxaliplatin? Pharmacogenomics J 2008; 8:315–20.
- Li X, Ding X, Adrian TE. Arsenic trioxide causes redistribution of cell cycle, caspase activation, and GADD expression in human colonic, breast, and pancreatic cancer cells. Cancer Invest 2004;22:389–400.
- Nakagawa Y, Akao Y, Morikawa H, Hirata I, Katsu K, Naoe T, . Arsenic trioxide-induced apoptosis through oxidative stress in cells of colon cancer cell lines. Life Sci 2002;70: 2253–69.