Abstract
Purpose. To verify the possibility of using Helical Tomotherapy to safely escalate dose to single or multiple highly radioresistant dominant intra-prostatic lesions (DILs) as assessed by functional magnetic resonance imaging (MRI). Material. In seven intermediate/high risk patients, T2WI, T1WI and DWI MRI imaging showed evidence of one DIL in four patients and two DILs in three patients in the peripheral zone of the prostate. The planning strategy was to deliver median doses of 80, 90, 100 and 120 Gy to PTVDIL while delivering 71.4 Gy/28 fractions (EQD2=75 Gy) to the remaining portion of PTV. A higher priority was assigned to rectal constraints relative to DIL coverage. Rectal NTCP calculations were performed using the most recently available model data. Results. The median dose to DIL could safely be escalated to at least 100 Gy (EQD2,α/β=10=113 Gy) without violating safe constraints for the organs at risk. Typical rectal NTCP values were around or below 1–3% for G3 toxicity and 5–7% for G2–G3 toxicity. For the 100 Gy DIL dose boost strategy, mean D95% of DIL and PTVDIL were 98.8 Gy and 86.7 Gy, respectively. The constraints for bladder, urethra and femoral heads were always respected. Conclusions. IGRT by Helical Tomotherapy may permit the safe escalation of EQD2,α/β=10 to at least 113 Gy to DILs without significantly increasing rectal NTCP compared to plans without dose escalation. A Phase I–II clinical study is warranted.
The assumption that prostate cancer is a prevalently multi-focal disease [Citation1,Citation2] is quite solid such that the irradiation of the whole prostate is traditionally considered as mandatory by radiation oncologists. Dose escalation to the whole prostate has been proven to impact on biochemical and distant metastasis failure rates [Citation3]. However, even in the IMRT-IGRT era, this approach is subject to limitations due to the proximity of a number of organs at risk, primarily the rectum. On the other hand, the evidence that local relapse after radiotherapy mostly originates at the original tumor location [Citation4,Citation5] at the level of one or more dominant intra-prostatic lesions (DILs) suggests a possible alternative to “whole-prostate dose escalation” through selective dose escalation to the DILs while maintaining a sufficiently high dose to the remaining portion of the prostate. With this approach the dose to critical organs at risk (OAR) is expected to be reduced if compared to the whole organ escalation approach [Citation6].
A number of pre-requisites need to be satisfied in order to carry out a safe “DIL-escalation” approach: a) availability of an advanced radiotherapy technique such as IMRT that allows an increase in the conformity of the dose to the tumor volume while minimizing doses to healthy organs; b) combination with image guided techniques (IGRT) that are able to minimize geometric uncertainties caused by organ motion/deformation of the target and critical structures and day-to-day set-up variation; c) the availability of appropriate imaging tools able to enhance DIL, possibly with high sensitivity/specificity.
Few investigations have highlighted the evidence of radioresistant regions within the prostate using a number of methods [Citation7–9] with few investigations suggesting a correlation between pre-radiotherapy tumor hypoxia and local failure [Citation10,Citation11]. Several MRI techniques have been explored to detect intra-prostatic lesions: MRI spectroscopy [Citation12], dynamic contrast enhanced MRI [Citation12,Citation13], T2 weighted MRI [Citation13,Citation14] and diffusion weighted magnetic resonance imaging (DW-MRI) [Citation14,Citation15] have been investigated as appropriate imaging modalities to visualize DILs, although the reports dealing with the accuracy of these functional imaging modalities in detecting areas of hypoxia or proliferation are still limited [Citation16].
Several recent studies have demonstrated that DWI can help differentiate malignant and benign prostatic tissue on the basis of lower apparent diffusion coefficient (ADC) values of carcinoma compared with normal tissue [Citation17–19]. The reduction of diffusion in prostate cancer is believed to be related to the more cellular environment of neoplastic tissue which restricts water molecule movement in extracellular space.
The expected impact of dose escalation on DIL critically depends on the assumptions concerning the radioresistance of clonogens within and outside the DILs [Citation20–23].
In the hypothesis that local control is mainly due to the control of radioresistant, possibly hypoxic clonogens within the DIL, 2 Gy equivalent (EQD2) doses as high as 100–120 Gy have been suggested as necessary to sterilize most tumors [Citation22]; the delivery of such “ultra-high” doses remains a challenge even with highly sophisticated IMRT-IGRT systems due to the proximity of various organs at risk, primarily the rectum and secondarily bladder and urethra.
The purpose of the current study is to explore the possibility of using Helical Tomotherapy to safely escalate the EQD2 within the DIL to these ultra-high levels. Because the close relationship between DIL and the anterior rectal wall represents the main limitation to DIL dose escalation, rectal NTCP calculations were also performed using the most recently available models [Citation24–28] in order to investigate whether substantial dose escalation is feasible without significantly increasing the risk of rectal complications.
Materials and methods
Patients
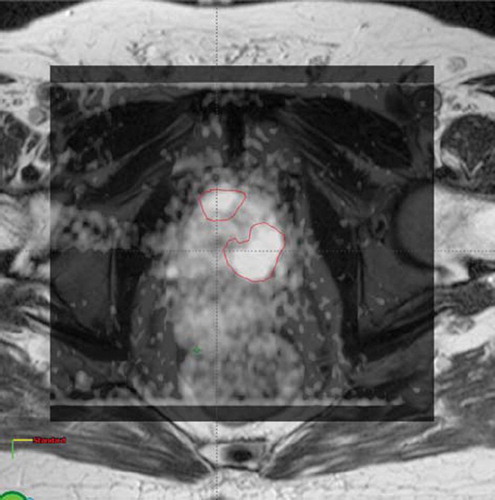
Seven intermediate/high risk patients, submitted to T2WI, T1WI and DWI, showed evidence of DIL in the peripheral zone (one DIL in four patients and two in three patients). As an example, shows DWI superimposed to T2WI for one patient with two DILs.
Median patient age was 76 years (range: 62–80 years). Median PSA was 6.26 ng/ml (range: 4.8–19.7 ng/ml). A summary of clinical and volumetric data is shown in .
Table I. Age, PSA level, Cleason score, targets and rectum volumes for the seven patients.
CT imaging and MRI
The planning CT scan was obtained with a 4-mm slice thickness (the most used thickness in our clinical practice which fits with the MVCT normal acquisition mode) with a multislice CT scanner (GE Medical Systems).
MRI scans were performed on a 1.5-Tesla MRI scanner (Achieva; Philips Medical Systems) using phase-array coil (number of channels = 4). The entire prostate gland and seminal vesicles were covered by axial T1-weighted imaging (T1WI; repetition time, 400 ms; echo time, 14 ms) and axial, sagittal and frontal T2-weighed imaging (T2WI; repetition time, 6 199 ms, echo time, 120 ms). These conventional images were obtained with a 3-mm slice thickness, 180–200 mm FOV, and matrix size of 336x284. Axial isotropic DWI parameters were repetition time, 2.5 ms; echo time, 80 ms; flip angle, 90°; slice thickness, 6 mm; b-values, 0, 600 and 1 200 s/mm2; matrix, 128x80; and FOV, 160 mm.
The post-processing of DWI images with b-value of 0 and 1 200 s/mm2 was performed on a Viewforum Philips medical system workstation where it was possible to generate Apparent Diffusion Coefficient (ADC) maps calculating the ADC value in each pixel of each slice. ADC values were measured manually drawing regions of interests (ROIs) on ADC maps in the centre of each visible DIL and inside the prostate gland.
Patients were imaged in supine position on a flat couch. CT and MRI images were rigidly matched using the Eclipse treatment planning software. Initially, a fully automatic rigid registration based on bone anatomy, using mutual information algorithm, was carried out; then the match was manually adjusted by a physician on the prostate. Patients were instructed to undergo planning CT and MRI with a comfortably full bladder and with empty rectum. If rectum volume was >100 cm3 the planning CT\MRI was repeated. These procedures were found to reduce prostate motion: the systematic and random errors for organ motion, relative to bone anatomy, were 0.44/1.3 mm and 0.34/0.9 mm respectively without and with the application of post MVCT rectal emptying procedures [Citation31]. All images of the two examinations were carried out within one hour of each other to minimize any potential impact of different rectum/bladder filling on prostate position.
Target volume and organ at risk delineation
The prostate and seminal vesicles were defined as the clinical target volumes (CTV) and the corresponding planning target volume (PTV) was generated by adding 8-8-10 mm anisotropic margins to the CTV (in lateral, anterior-posterior and cranial-caudal direction, respectively) as routinely done at our Institution [Citation29,Citation30]. These margins were similar to the “safe” ones applied in 3DCRT. Rectum, bladder, urethra (delineated on MRI images) and femoral heads were outlined as OARs. The rectum was contoured from the anus to the recto-sigmoid junction and femoral heads+femurs were contoured up to the ischial tuberosities level. DILs were contoured by an expert radiologist (F.D.C.) who was aware, during the evaluation of images, of tumor characteristics (including ultrasounds, digital rectal examination and biopsy results) and was able to switch between T2WI, T1WI, DWI and CT. The addition of DWI to T2WI and T1WI improves the detectability of DILs; the sensitivity of such composite techniques has been reported to be around to or higher than 80% [Citation15]. The PTV corresponding to DIL (PTVDIL) was generated by 0.5 cm automatic expansion; this value was suggested as the minimum margin safely achievable when on-line MVCT correction is applied, to take into account intra-fraction and intrinsic uncertainty of the visualization of the prostate with MVCT [Citation29,Citation31]: in the context of daily IGRT, the major component of the residual error is random such that the impact on the actually delivered dose on the rectum and on PTV (where the dose gradient is steeper) is expected to be negligible in a more than 20 fractions scenario [Citation32].
Fused MRI-CT images were used for Tomotherapy planning.
Tomotherapy planning optimization and dose constraints
The planning strategy used in this study derives from a Phase I–II moderately hypofractionated simultaneous integrated boost trial in progress at our institute [Citation30]. In the current study, for simplicity, we excluded pelvic lymph nodes from planning optimization and considered prostate + seminal vesicles as a single CTV. Tomotherapy planning optimization was performed using a field dimension of 2.5 cm, a pitch of 0.3 and a modulation factor of 4. The corresponding irradiation times were generally in the range of 7–8 min.
The planning strategy used in the study was to deliver 71.4 Gy (2.55 Gy/fr; EQD2,α/β=10=75 Gy) to PTV(prostate+seminal vescicles) outside the PTVDIL while delivering a median dose to PTVDIL equal to 80 Gy (2.86 Gy/fr; EQD2,α/β=10=86 Gy), 90 Gy (3.21 Gy/fr; EQD2,α/β=10=99 Gy), 100 Gy (3.57 Gy/fr; EQD2,α/β=10=113 Gy) and 120 Gy (4.29 Gy/fr; EQD2,α/β=10=143 Gy), to the DIL, in 28 fractions. There is currently a wide variation of α/β values for prostate cancer reported in the literature with the exact value still unknown. Brenner et al. [Citation33] estimated α/β values ranging from 1 to 5 Gy with an average value of 1.5 Gy. The main limit of all these values for the fractionation sensitivity of prostate cancer is that they consider outcomes derived from brachytherapy and external beam RT. Valdagni et al. [Citation34], comparing standard fractionation (median dose of 74 Gy, 2 Gy/fraction, delivered in 51 days) and hyperfractionation (median dose of 79.2 Gy, 2x1.2 Gy/fraction daily, delivered in 45 days) results on 330 patients, estimated an α/β=8.3 Gy. In a recent study, Nahum et al. [Citation22] demonstrated that neither α/β, nor the density of clonogenic cells needs to be extremely low to explain prostate cancer response to brachytherapy and external beam RT. They incorporated new hypoxia measurements from prostate cancer into the TCP model and produced a good fit for brachytherapy and external beam RT outcomes using α/β=8.4 Gy for normal cells and α/β=15.5 Gy for the hypoxic fraction. Therefore, an α/β ratio of 10 was chosen consistently with a high radioresistance of the clonogens inside the DIL [Citation22].
As the main limitation to dose escalation was rectum dose, due to the lack of knowledge about the risk of late rectal sequelae (as well as for other OARs such as urethra and bladder) resulting from very high doses (>80–90 Gy), the chosen strategy was to satisfy “safe” rectal constraints derived from external radiotherapy corrected by the linear-quadratic model (V65.5 Gy<20%, V68.5 Gy<5% [Citation29,Citation31,Citation35]) in combination with constraints translated from brachytherapy experience applied to the tail of rectal DVH (V80 Gy [EQD2;α/β=3=94 Gy]<0.1 cm3; V75 Gy [EQD2;α/β=3= 85 Gy]<1 cm3; V70 Gy [EQD2;α/β=3= 77 Gy] <2 cm3) [Citation36,Citation37]. Having once satisfied these constraints, the planner tried to reduce the dose as low as possible to the fraction of rectum outside the PTV. Constraints for bladder (V75 Gy<0.1 cm3 and “as low as possible outside the PTV”), urethra (V80 Gy<1 cm3, V90 Gy<0.1 cm3 [Citation38,Citation39]), bulbus of penis (V52 Gy<50% [Citation40]) and femoral heads (Dmax<40 Gy) were applied as well. A summary of the applied constraints is shown in .
Table II. Main constraints for the different organs at risk.
For all patients, the dose was prescribed as median dose to the PTV. Concerning PTV, the goal was to deliver more than 95% of the prescribed dose to more than 95% of the volume while keeping dose homogeneity as high as possible. PTVDIL coverage, instead, was constrained by OAR sparing.
DVH was used to evaluate the dose distribution of PTV, PTVDIL, DIL, rectum, bladder, urethra and femoral heads for the different planning strategies and for each patient. Rectal NTCP calculations were performed using the most recently available models [Citation24–28] in order to assess whether dose escalation may cause an increased risk of rectal toxicity; rectum NTCP values have been estimated with six different sets of NTCP model parameters reported in literature ([Citation24–28], see ).
Table III. NTCP model parameters. n is a parameter which describes the importance of volume effect; D50 is the dose that causes 50% probability of injury and m is the slope of response curve at D50.
Statistical tests
The Wilcoxon matched-pair signed-rank test for non-parametrically distributed data was used to compare rectum NTCP between plans without DIL boost and plans with DIL dose escalation. Statistical tests were carried out using statistical software (SPSS, version 17). Values of p<0.05 were considered to denote significant differences.
Results
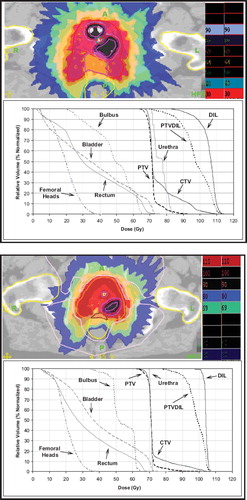
For all patients the median dose to PTVDIL could be escalated to at least 100 Gy (EQD2,α/β=10=113 Gy) without violating the constraints reported in for OARs; the escalation to 120 Gy (EQD2,α/β=10=143 Gy) was possible for three patients who did not show any PTVDIL-rectum overlap; for the remaining four patients, the increase of dose to significantly large portions of the DIL would result in important violations of the rectal constraints. shows examples of Tomotherapy planning with dose escalation up to 100 Gy to a double (a) and a single (b) DIL.
Figure 2. Examples of Tomotherapy planning with dose escalation up to 100 Gy to a double and single DIL: mean DIL dose is 100 Gy (EQD2=122 Gy, α/β=3). The remaining prostate portion receives 71.4 Gy (28 fractions).
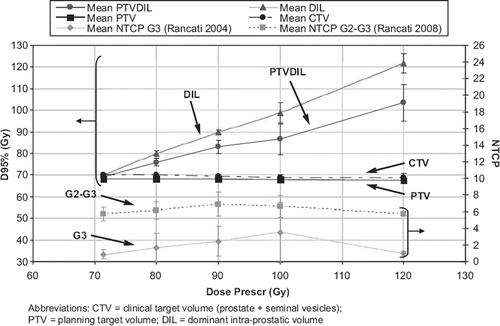
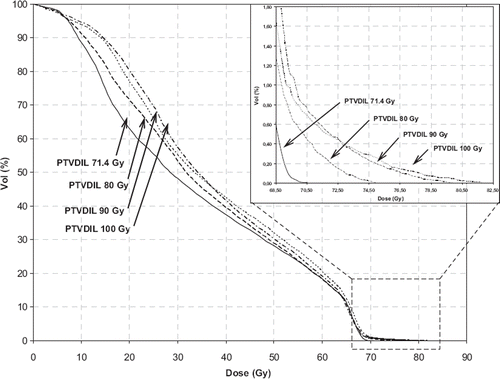
summarizes mean rectal NTCP values, calculated using different NTCP model parameters for the seven patients and for the different planning strategies. It can be observed that rectal NTCP values were not significantly increased (p-value>0.11) compared to planning without DIL boost (). Mean NTCP values were about 0.9–3.5% for G3 toxicity and about 5–7% for G2–G3 toxicity (). NTCP values at 120 Gy DIL dose were lower than at 100 Gy because they refer to the three patients with favourable anatomy (no PTVDIL-rectum overlap). As shown in , for the 100 Gy DIL dose boost strategy (the highest median DIL dose delivered to all patients), the mean/median values of the dose received by 95% of volume (D95%) of DIL and PTVDIL were 98.8/100.6 Gy and 86.7/85.7 Gy respectively. shows that PTVDIL dose escalation slightly increases the rectal dose at low dose values and in the tail of DVH; all rectum dose-volume values were within constraints. Mean values of V40Gy, V50Gy and V60Gy for the rectum were 39.9%, 29.3% and 18.6% for selective boosting plans and 37.6%, 28.3% and 18.5% for plans without dose escalation. The absolute differences between NTCP for planning with and without escalation are generally within 1–2%, depending on the applied model. These differences suggest that, even in case of a large population where they could prove statistically significant, they would be clinically irrelevant. This is a direct consequence of the optimization approach followed.
Table IV. Mean rectal NTCP/EUD values for the seven patients and for the different planning. strategies.
The ADC values for the seven patients are presented in . A significant difference of mean ADC values between the DILs (0.66 ± 0.0731023 mm2/s) and prostate tissues (1.12± 0.1331023 mm2/s) were found (p<0.0001).
Table V. Apparent diffusion coefficient (ADC) values for the seven patients measured in the visible intra-prostatic lesions (DILs) and inside prostate gland.
Discussion
In this study, we demonstrated the potential of Helical Tomotherapy to selectively increase the dose to single or multiple high risk regions within prostate gland (DILs), detected by functional imaging techniques such as T2WI, T1WI and DWI. Based on our findings, substantial dose escalation (at least up to 100 Gy corresponding to EQD2,α/β=10=113Gy) while treating the rest of the gland to 71.4 Gy (EQD2,α/β=10=75 Gy), without increasing the likelihood of toxicity in the adjacent normal organs seems to be feasible with a large (>85%) portion of PTVDIL (using a 5 mm margin) receiving more than 95% of the prescribed dose and almost the whole DIL receiving the prescribed dose. The concept of intra-prostatic boost with a dose >>70 Gy, while maintaining the dose to the rest of prostate at a sufficiently high value, is based on the Cellini et al. [Citation4] study on 118 prostate patients irradiated at 65–70 Gy. This study revealed that all 12 observed recurrences within the prostate originated in the primary tumor site. More recently, Pucar et al. [Citation5] corroborated this statement by clearly showing that the intra-prostatic relapse after external radiotherapy occurs at the site of primary dominant lesions by step-section pathology specimens after salvage radical prostatectomy. In an early work, Pickett et al. [Citation42] reported on the possible advantages of incorporating MRI into a static field prostate IMRT plan. In their study the feasibility of treating a single lobe to 90 Gy by MRSI guidance was demonstrated. Nutting et al. [Citation41] performed a study in six patients in which the DIL volume was defined based on the information derived from prostatectomy. Comparing IMRT plans with whole homogenous prostate irradiation and plans with a 20 Gy additional boost to the DIL, the estimated TCP increased from 64.4 to 95.6%. Van Lin et al. [Citation12] performed a similar analysis on five patients finding that, for all planning strategies, it was possible to escalate the DIL dose without reducing margins around CTV.
On the other hand, little clinical experience of dose escalation on DILs has been reported to date, mainly with brachytherapy [Citation38,Citation43].
Concerning external radiotherapy, probably the most important challenge of this approach is represented by a lack of knowledge about rectal side-effects (and of other OARs such as urethra and bladder) at very high doses (>85–90 Gy); as reported by De Meerleer et al. [Citation44] in their preliminary clinical experience on 15 patients, the dose escalation to T2W MRI-based DIL was limited to only about 2 Gy by the hard constraints for the rectal dose applied by the authors. Preliminary findings from a Phase I study escalating the dose to 95 Gy (2.25 Gy/fr) to DIL (+3 mm margin) were recently reported [Citation45]: acute toxicity data of only three patients were reported, showing no Grade 3 or higher gastro-intestinal or genito-urinary toxicity. The study should continue to escalate the dose with the last step planned to be 152 Gy in 42 fractions.
More recently, Fonteyne et al. [Citation46] reported the experience of moderate dose escalation on DILs, defined by MRI and/or MRS, to about 82 Gy while delivering 78 Gy to the remaining part of the prostate on 118 patients. The incorporation of DILs into treatment planning resulted in a clinically deliverable SIBIMRT with an acute toxicity profile comparable to that recorded in the population of 112 patients without evidence of DILs.
Based on the assumption that the DIL may be characterized by the presence of hypoxic cells, TCP models including hypoxia [Citation21,Citation22] suggest that the dose should be escalated to ultra-high EQD2 (>100–120 Gy) in order to sterilize cancer cells in a largely hypoxic region. However, hypothesizing hypoxia as the cause of the radioresistance in the DILs is still far from being fully confirmed, although some authors have found an interesting correlation between resistant cancer and hypoxia markers [Citation10,Citation11]; on the other hand, spectroscopic and morphological alterations found with MRI suggest that DILs are highly resistant foci of cancer cells without clear evidence of hypoxia [Citation9].
Further investigations are necessary to better assess potentials and limits of using IGRT to boost the dominant intra-prostatic lesion. Due to the proximity of DILs to the rectal wall, an as accurate as possible estimate of the risk of complications for rectum should be assessed as in our planning investigation.
Rectal toxicity has been recently modelled with NTCP/EUD models in a number of independent investigations involving in total more than 3 000 patients [Citation24–28]; these studies showed the prevalent seriality of the rectum when considering rectal bleeding as the end-point. The combination of external beam based NTCP models with dose-volume constraints translated from the brachytherapy experience and applied to the tail of the DVH, as in our study, seems to be one of the possible “safe” strategies for dose escalation. The DIL location, namely the proximity of DIL to rectum influences PTVDIL coverage. In fact, the closer the DIL is to the rectum the lower D95% of PTVDIL will be in order to satisfy the rectal dose constraints. For patients with DIL not overlapped with rectum it was possible to safely escalate the dose up to 120 Gy (EQD2,α/β=10=143 Gy).
An important point of DIL escalation approaches is the reliability of the imaging technique used to identify the intra-prostatic lesions. The combined technique used in our study was reported to be highly sensitive [Citation15,Citation16]. However, the impact of the intrinsic accuracy limitation of imaging techniques (namely sensitivity and specificity) should be investigated in order to understand its influence on DIL dose escalation strategies.
ADC measurements from DWI sequences performed with b=0 and 1 200 mm2/s showed that the ADC value measured inside DILs was significantly lower than that inside the prostate; similar ADC values were recently obtained by Woodfield et al. [Citation47]. Decreased ADC values inside DILs relative to the prostate gland has been well documented to be related to a significant reduction in the diffusion properties of water protons in prostate. In fact, the diffusion characteristics of any biologic tissue are based on the relative combination of water proton movement in the extracellular environment, across cell membranes, and within the cells of that tissue. Any change in tissue architecture such as an increase in the proportion of intracellular to extracellular water protons, which occurs with the replacement of less cellular normal prostate tissue with more highly cellular neoplastic tissue, results in more restricted movement of water protons and therefore a reduction of measured ADC value.
Image-guided radiotherapy techniques are clearly required in order to accurately deliver these treatments. The accuracy of the IGRT techniques as well as intra-fraction motion should be incorporated into the margin around the DIL. In our Tomotherapy scenario it was demonstrated that the margin could with difficulty be reduced to less than 5 mm [Citation29,Citation31]: for this reason we avoided applying a smaller margin for PTVDIL (i.e., 3 mm), which could introduce a significant risk of missing the target. Taking into account the very low acute and early late toxicity for patients treated in our institution [Citation30] with simultaneous integrated boost approach, it was decided to keep, for PTV, the “safe” margins used for 3DCRT.
The results of this pre-clinical study support the activation of a Phase I–II clinical trial including dose painting to PTVDILs up to 100 Gy in 28 fractions.
An investigation regarding the estimates of the gain of TCP with this approach and how this gain may depend on the presence and location of resistant cells is in progress. The estimate of the expected gain from DIL-escalated planning is of paramount importance to better assess future Phase III trials after the completion of Phase I–II clinical investigations.
Declaration of interest: No concflict of interest exists for all authors.
References
- Djavan B, Milani S, Remzi M. Prostate biopsy: Who, how and when. An update. Can J Urol 2005;12(Suppl 1):44–8.
- Isbarn H, Karakiewicz PI, Vogel S, Jeldres C, Lughezzani G, Briganti A, . Unilateral prostate cancer cannot be accurately predicted in low-risk patients. Int J Radiat Oncol Biol Phys 2010;77:784–7.
- Viani GA, Stefano EJ, Afonso SL. Higher than-conventional radiation doses in localized prostate cancer treatment: A meta-analysis of randomized, controlled trials. Int J Radiat Oncol Biol Phys 2009;74:1405–18.
- Cellini N, Morganti AG, Matteucci GC, Valentini V, Leone M, Luzi S, . Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: Implications for conformal therapy planning. Int J Radiat Oncol Biol Phys 2002;53:595–9.
- Pucar D, Hricak H, Shukla-Dave A, Kuroiwa K, Drobnjak M, Eastham J, . Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: Magnetic resonance imaging and step-section pathology evidence. Int J Radiat Oncol Biol Phys 2007;69:62–9.
- Kim Y, Tomè WA. Is it beneficial to selectively boost high-risk tumor subvolumes? A comparison of selectively boosting high-risk tumor subvolumes versus homogeneous dose escalation of entire tumor based on equivalent EUD plans. Acta Oncol 2008;47:906–16.
- Parker C, Milosevic M, Toi A, Sweet J, Panzarella T, Bristow R, . Polarographic electrode study of tumor oxygenation in clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2004;58:750–7.
- Rasey JS, Koh WJ, Evans ML, Peterson LM, Lewellen TK, Graham MM, . Quantifying regional hypoxia in human tumors with positron emission tomography of [18F] fluoromisonidazole: A pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys 1996;36:417–28.
- Villeirs GM, De Meerleer GO, De Visschere PJ, Fonteyne VH, Verbaeys AC, Oosterlinck W, . Combined magnetic resonance imaging and spectroscopy in the assessment of high grade prostate carcinoma in patients with elevated PSA: A single-institution experience of 356 patients. Eur J Radiol 2010 (in press).
- Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Greenberg RE, Stobbe C, . Hypoxic prostate/muscle pO2 ratio predicts for biochemical failure in patients with prostate cancer: Preliminary findings. Urology 2002;60:634–9.
- Vergis R, Corbishley CM, Norman AR, Bartlett J, Jhavar S, Borre M, . Intrinsic markers of tumour hypoxia and angiogenesis in localised prostate cancer and outcome of radical treatment: A retrospective analysis of two randomised radiotherapy trials and one surgical cohort study. Lancet Oncol 2008;9:342–51.
- Van Lin ENJT, Futterer JJ, Heijmink SWTPJ, Van Der Vight LP, Hoffmann AL, Van Kollenburg P, . IMRT boost dose planning on dominant intraprostatic lesions: Gold marker-based three-dimensional fusion of CT with dynamic contrast-enhanced and 1H-spectroscopic MRI. Int J Radiat Oncol Biol Phys 2006;65:291–303.
- Moman MR, Van Den Berg CAT, Boeken Kruger AE, Battermann JJ, Moerland MA, Van Der Heide UA, . Focal salvage guided by T2-weighted and dynamic contrast-enhanced magnetic resonance imaging for prostate cancer recurrences. Int J Radiat Oncol Biol Phys 2010;76:741–6.
- Haider MA, Van der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, Lockwood G, . Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol 2007;189:323–8.
- Kajihara H, Hayashida Y, Murakami R, Katahira K, Nishimara R, Hamada Y, . Usefulness of diffusion-weighted imaging in the localization of prostate cancer. Int J Radiat Oncol Biol Phys 2009;74:399–403.
- Groenendaal G, van den Berg CAT, Korporaal JG, Philippens MEP, Luijten PR, van Vulpen M, . Simultaneous MRI diffusion and perfusion imaging for tumor delineation in prostate cancer patients. Radiother Oncol 2010;95:185–90.
- Kim CK, Park BK, Han JJ, Kang TW, Lee HM. Diffusion-weighted imaging of the prostate at 3 T for differentiation of malignant and benign tissue in transition and peripheral zones: Preliminary results. J Comput Assist Tomogr 2007;31:449–54.
- Sato C, Naganawa S, Nakamura T, Kumada H, Miura S, Takizawa O, . Differentiation of noncancerous tissue and cancer lesions by apparent diffusion coefficient values in transition and peripheral zone of the prostate. J Magn Reson Imaging 2005;21:258–62.
- Kumar V, Jagannathan NR, Kumar R, Das SC, Jindal L, Thulkar S, . Correlation between metabolite ratios and ADC values of prostate in men with increased PSA level. Magn Reson Imaging 2006;24:541–8.
- Tomé WA, Fowler JF. Selective boosting of tumor subvolumes. Int J Radiat Oncol Biol Phys 2000;48:593–9.
- Popple RA, Ove R, Shen S. Tumor control probability for selective boosting of hypoxic subvolumes, including the effect of reoxygenation. Int J Radiat Oncol Biol Phys 2002;54:921–7.
- Nahum AE, Movsas B, Horwitz EM, Stobbe CC, Chapman JD, . Incorporating clinical measurements of hypoxia into tumor local control modelling of prostate cancer: Implications for the α/β ratio. Int J Radiat Oncol Biol Phys 2003;57: 391–401.
- Niyazi M, Bartenstein P, Belka C, Ganswindt U. Choline PET based dose-painting in prostate cancer – Modelling of dose effects. Radiat Oncol 2010;5:23.
- Rancati T, Fiorino C, Gagliardi G, Cattaneo GM, Sanguineti G, Casanova Borca V, . Fitting late rectal bleeding data using different NTCP models: Results from an Italian multi-centric study (AIROPROS0101). Radiother Oncol 2004;73:21–32.
- Rancati T, Fiorino C, Vavassori V, Baccolini M, Bianchi C, Foppiano F, . Late rectal bleeding after conformal radiotherapy for prostate cancer: NTCP modelling. Radiother Oncol 2008;88:S332–3.
- Peeters STH, Hoogeman MS, Heemsbergen WD, Hart AAM, Koper PCM, Lebesque JV, . Rectal bleeding, fecal incontinence, and high stool frequency after conformal radiotherapy for prostate cancer: Normal tissue complication probability modeling. Int J Radiat Oncol Biol Phys 2006;66:11–9.
- Söhn M, Yan D, Liang J, Meldolesi E, Vargas C, Alber M, . Incidence of late rectal bleeding in high-dose conformal radiotherapy of prostate cancer using equivalent uniform dose-based and dose-volume-based normal tissue complication probability models. Int J Radiat Oncol Biol Phys 2007;67: 1066–73.
- Tucker SL, Dong L, Bosch WR, Michalski J, Winter K, Lee AK, . Fit of a generalized Lyman Normal tissue complication probability (NTCP) model to grade≥2 late rectal toxicity data from patients treated on protocol 94-06. Int J Radiat Oncol Biol Phys 2007;69:S8–9.
- Fiorino C, Alongi F, Broggi S, Cattaneo GM, Cozzarini C, Di Muzio N, . Physics aspects of prostate tomotherapy: Planning optimization and image guidance issue. Acta Oncol 2008;47:1309–16.
- Di Muzio N, Fiorino C, Cozzarini C, Alongi F, Broggi S, Mangili P, . Phase I-II study of hypofractionated simultaneous integrated boost with tomotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2009;74:392–8.
- Fiorino C, Di Muzio N, Broggi S, Cozzarini C, Maggiulli E, Alongi F, . Evidence of limited motion of the prostate by careful emptying of the rectum as assessed by daily MVCT image-guidance with Helical Tomotherapy. Int J Radiat Oncol Biol Phys 2008;71:611–7.
- Rijkhorst EJ, Lakeman A, Nijkamp J, De Bois J, van Herk M, Lebesque JV, . Strategies for online organ motion correction for intensity-modulated radiotherapy of prostate cancer: Prostate, rectum, and bladder dose effects. Int J Radiat Oncol Biol Phys 2008;75:1254–60.
- Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 1999;43:1095–101.
- Valdagni R, Italia C, Montanaro P, Lanceni A, Lattuada P, Magnani T, . Is the alpha-beta ratio of prostate cancer really low? A prospective, non-randomized trial comparing standard and hyperfractionated conformal radiation therapy. Radiother Oncol 2005;75:74–82.
- Fellin G, Fiorino C, Rancati T, Vavassori V, Baccolini M, Bianchi C, . Clinical and dosimetric predictors of late rectal toxicity after conformal radiation for localized prostate cancer: Results of a large multicenter observational study. Radiother Oncol 2009;93:197–202.
- Koom WS, Sohn DK, Kim JY, Kim JW, Shin KH, Yoon SM, . Computed tomography-based high-dose-rate intracavitary brachytherapy for uterine cervical cancer: Preliminary demonstration of correlation between dose-volume parameters and rectal mucosal changes observed by flexible sigmoidoscopy. Int J Radiat Oncol Biol Phys 2007;68:1446–54.
- Georg P, Kirisits C, Goldner G, Dörr W, Hammer J, Pötzi R, . Correlation of dose volume parameters, endoscopic and clinical rectal side effects in cervix cancer patients treated with definitive radiotherapy including MRI based brachytherapy. Radiother Oncol 2009;91:173–80.
- Kim Y, Hsu ICJ, Lessard E, Kurhanewicz J, Noworolski SM, Pouliot J, . Class solution in inverse planned HDR prostate brachytherapy for dose escalation of DIL defined by combined MRI/MRSI. Radiother Oncol 2008;88:148–55.
- Martinez A, Linares LA, Wallace M, Galelae R. Phase II prospective non-randomized trial of the use of conformal high dose rate brachytherapy alone for the treatment of favorable stage adenocarcinoma of the prostate. Prostate Brachytherapy International Group. Study HDR-01, 2007.
- Roach M, Winter K, Michalski JM, Cox JD, Purdy JA, Bosch W, . Penile bulb dose and impotence after three-dimensional conformal radiotherapy for prostate cancer on RTOG 9406: Findings from a prospective, multi-institutional, phase I/II dose-escalation study. Int J Radiat Oncol Biol Phys 2004; 60:1351–6.
- Nutting CM, Corbishley M, Sanchez-Nieto B, Cosgrove VP, Webb S, Dearnaley D, . Potential improvements in the therapeutic ratio of prostate cancer irradiation: Dose escalation of pathologically identified tumour nodules using intensity modulated radiotherapy. Br J Radiol 2002;75:151–61.
- Pickett B, Vigneault E, Kurhanewicz J, Verhey L, Roach M. Static field intensity modulation to treat a dominant intra-prostatic lesion to 90 Gy compared to seven field 3-dimensional radiotherapy. Int J Radiat Oncol Biol Phys 1999;44:921–9.
- Ares C, Popowski Y, Pampallona S, Nouet P, Dipasquale G, Bieri S, . Hypofractionated boost with high-dose-rate brachytherapy and open magnetic resonance imaging-guided implants for locally aggressive prostate cancer: A sequential dose-escalation pilot study. Int J Radiat Oncol Phys 2009; 75:656–63.
- De Meerleer G, Villeirs G, Bral S, Paelinck L, De Gersem W, Dekuyper P, . The magnetic resonance detected intraprostatic lesion in prostate cancer: Planning and delivery of intensity-modulated radiotherapy. Radiother Oncol 2005;75:325–33.
- Housri N, Ondos J, Choyke PL, Barbara A, Ning H, Citrin D, . Tumor nodule location predicts the feasibility of intraprostatic high-dose irradiation in men with localized prostate cancer. Int J Radiat Oncol Biol Phys 2009;75(Suppl1):S14–4.
- Fonteyne V, Villeirs G, Speleers B, De Neve W, De Wagter C, Lumen N, . Intensity modulated radiotherapy as primary therapy for prostate cancer: Report on acute toxicity after dose escalation with simultaneous integrated boost to intraprostatic lesion. Int J Radiat Oncol Biol Phys 2008;72:799–807.
- Woodfield CA, Tung GA, Grand DJ, Pezzullo JA, Machan JT, Renzulli JF. Diffusion-weighted MRI of peripheral zone prostate cancer: Comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. AJR Am J Roentgenol 2010;194:W316–22.