Abstract
The aim of the study was to evaluate the feasibility of tailored and dose-dense epirubicin and cyclophosphamide followed by docetaxel as adjuvant breast cancer therapy. Material and methods. Patients with node-positive breast cancer received either four cycles of biweekly and tailored EC (epirubicin 38-60-75-90-105-120 mg/m2, cyclophosphamide 450-600-900-1200 mg/m2) followed by four cycles of docetaxel (60-75-85-100 mg/m2) (arm A) or the same regimen with fixed doses (E90C600 + 4 → T75 + 4) (arm B) or docetaxel, doxorubicin and cyclophosphamide (T75A50C500) every three weeks for six cycles (arm C). All patients received G-CSF support and prophylactic ciprofloxacin. Results. One-hundred and twenty-four patients were randomised in the study. In the A, B and C arm, 17% 19% and 3% of the patients had one or more cycles delayed due to side-effects whereas 24%, 5% and 15% experienced a grade 3 infection or febrile neutropenia. After the introduction of an extra week between the EC and T parts in the A and B arms, grade 3 hand-foot-skin reactions were reduced from 5 to 0.2%. Twenty-nine percent (A and B) and 20% (C) of the patients were hospitalised due to side-effects. Discussion. Dose-dense and tailored EC/T can be given with manageable toxicity and is after adjustment presently studied in the phase III Panther trial.
Despite improvement in overall survival of breast cancer with adjuvant polychemotherapy including anthracyclines and subsequently taxanes, the risk of relapse remains significant, especially in lymph-node positive disease [Citation1–8]. Various dose and schedule strategies have been explored to overcome problems of relative or absolute drug resistance that might contribute to disease relapse. There is marked inter-patient variation in systemic exposure for the compounds in the standard adjuvant regimens despite that doses have been adjusted to body surface area [Citation9]. A tailored dosing concept, based on dosage aiming at inter-individual equivalent haematological toxicity, was explored in the Scandinavian Breast Group (SBG) 9401 study on breast cancer patients with very high risk for relapse [Citation10]. G-CSF (granulocyte colony stimulating factor) for 14 days supported tailored and dose escalated FEC (5-fluorouracil, epirubicin, cyclophosphamide) (cyclophosphamide to a maximal dose of 1.8 g/m2 per cycle) for nine cycles was compared with standard FEC followed by marrow supported high dose therapy in 525 randomised patients, resulting in statistically significantly fewer breast cancer relapses but no overall survival gain for the tailored arm. A major down-side was an increased risk for myelodysplasia (MDS) and acute leukemia (AML), ten patients developed this complication and that regimen is therefore not feasible [Citation10]. We then modified this regimen by reducing the number of cycles to six, decreasing the maximal cyclophosphamide dose to 1.2 g/m2 per cycle and the G-CSF use was shortened to a week. One-hundred and twenty-one patients were randomised to this modified tailored and dose escalated FEC regimen as part of the neo-adjuvant EORTC/P53 study which was closed in 2006. No MDS or AML have so far been recorded, however one patient developed a mantel cell lymphoma [Citation10]. The efficacy results were reported at ASCO 2010 [Citation11]. In the comparison between the taxane arm and the two FEC based arms, the five year progression-free survival was 65.1% in the docetaxel arm and 60.8 in the FEC-based arms (HR 0.85, stratified log rank test p = 0.035, pre-defined p-value = 0.02). Both FEC arms were slightly inferior compared to the docetaxel arm, the HR for French FE100C was 0.83 while the corresponding HR for the modified tailored and dose-escalated FEC was 0.91 (unpublished data from the EORTC). Finally, a tailored FEC regimen without G-CSF was studied in the prospective and randomised adjuvant study SBG 2000-1 compared with standard FE60C. These data are to be presented in San Antonio 2010.
Another biologically interesting concept is the dose dense strategy with chemotherapy administration every second week, which the use of cytokine G-CSF has permitted. A large phase III study compared two dose-dense regimens consisting of AC × 4 followed by paclitaxel x 4 every two weeks or sequential A = 4 → T = 4 → C × 4 every two weeks, both given with filgrastim support, with the same regimens given every third week [Citation12]. The AGO phase III trial compared a dose-dense (q2w) and dose-intense regimen consisting of sequential epirubicin, cyclophosphamide and paclitaxel with standard EC × 4 followed by paclitaxel × 4, q3w, in breast cancer patients with very high risk of relapse [Citation13]. Both these dose-dense regimens were considered feasible [12,13].
The primary aim of the present study was to evaluate the toxicity and feasibility of both tailored (possibility of dose escalation) and dose-dense (bi-weekly) EC/T. We also aimed to evaluate safety of the same regimen using only the dose-dense approach (dEC = 4 → dT = 4). As a standard arm for node positive breast cancer, TAC was chosen [Citation4].
Material and methods
Study objectives
The primary objective was to evaluate safety and feasibility of the treatment arms and the secondary objective was to evaluate the dose-intensity in the treatment arms.
Patient selection
This study enrolled female patients age 18–65, Eastern Cooperative Oncology Group performance status 0–1, with histological proven invasive primary breast cancer. The surgery should be radical with no tumour cells in the resection borders. Axillary dissection was done within 60 days of registration and at least one histological positive lymph node (>0.2 mm) among at least five (recommended 10) removed axillary nodes if receptor negative tumour or at least four positive lymph nodes if estrogen α or progesterone receptor positive tumour, was required. No proven distant metastases were accepted (negative pulmonary x-ray, bone-scintigram supplemented with conventional x-ray of hot-spots), and the patient should have normal liver and haematological function tests (if abnormal values the patient could be included if no metastases were demonstrated on CT or ultrasound of the liver and if the values did not preclude the possibility to safely deliver the cytotoxic agents in the study). No major cardiovascular morbidity (NYHA III and IV) was accepted. Exclusion criteria were locally advanced breast cancer, pregnancy or lactation, previous or concurrent malignancy at other sites, except basal cell carcinoma and/or squamous cell carcinoma in situ of the skin or cervix, other serious medical condition or peripheral neuropathy grade 2 or more. Patients with previous (>5 years) contra-lateral breast cancer with no objective findings of relapse could be included in the study.
After 93 patients had been randomised, an amendment of inclusion criteria was done, allowing patients, regardless of hormone receptor status, with at least one positive lymph node among at least five removed axillary nodes to be included.
Study design and treatment
The study protocol was approved by the ethics committee at the Karolinska Institute and by the Swedish Medical Product Agency. Patients received oral and written information followed by signed informed consent. Patients were randomly assigned to one of three treatment arms: four cycles of tailored epirubicin and cyclophosphamide every two weeks followed by four cycles of tailored docetaxel every two weeks (arm A) ( and ) or four cycles of fixed doses of epirubicin 90mg/m2 and cyclophosphamide 600 mg/ m2 every two weeks followed by four cycles of docetaxel 75 mg/m2 every two weeks or six cycles of standard TAC regimen (docetaxel 75 mg/m2, doxorubicin 50 mg/m2, cyclophosphamide 500 mg/m2) every three weeks. Patients in arm A at dose level 3 (cyclophosphamide 1200 mg/m2) received uromitexan (). Pre-and post chemotherapy medication included 5HT3 blockers and steroids. All patients received G-CSF, the first 93 patients as lenograstim 5 μg/kg day 4 to 11+ while the remaining patients received pegfilgrastim 6 mg on day 2, after each chemotherapy cycle. Haemoglobin, WBC, neutrophil and platelet values were measured each cycle at day 8, 11/12, 15 and at day 21 for the three-weekly TAC regimen. Prophylactic ciprofloxacin, 0.5 g + 2 daily, was given day 5–14 in all the three arms.
Table I. a. (top) Treatment arm A, dtEC and dtT dosages (mg/m2), ‘starting dose level; b. (bottom) Dose-modification schedules, arm A, dtEC (cycle 2–4) → dtT (cycle 6–8), in addition; diarrhoea grade ≥3 or stomatitis grade ≥3, reduce the dose one step the next cycle.
In arm A and B, an amendment was made after 124 of 842 cycles were administered, due to skin side-effects, resulting in a pause of one extra week between the EC and T cycles. All enrolled patients also received adjuvant radiotherapy, hormonal therapy and trastuzumab if clinically appropriate according to regional and national guidelines subsequent to their chemotherapy.
Assessment
Toxicity was assessed (through clinical Adverse Events) and graded according to National Cancer Institute Common Toxicity Criteria (NCI-CTC) version 3.0, at base-line and after each course.
Statistical considerations
The following criteria were set up as guidance of feasibility:
At least 90% of patients should receive the planned courses with ≤20% delay.
Less than 20% (8/40) of the patients in each treatment arm should be recorded with a grade III infection/febrile neutropenia and ≤5% (2/40) with a grade IV infection/febrile neutropenia according to CTC-NCI criteria.
Less than 20% of the patients should require hospitalisation due to toxicity (administered therapy).
Comparisons of distributions were made with Fisher's exact test for categorical data. For (distributional) comparisons of continuous variables, two-sample Wilcoxon rank-sum (Mann-Whitney) test and Kruskal-Wallis rank test were used.
Results
Patients
One-hundred and twenty-four patients (arm A: 42, arm B: 42, arm C: 40) were randomised in the study from December 2004 to May 2006. Ten Swedish centres participated in the study enrolling between 5–39 patients per centre. One Icelandic centre was opened but did not recruit any patients. Patients´ baseline characteristics are summarised in .
Table II. Baseline characteristics of randomised patients.
Administered treatment
Number of cycles given. Forty two, 41 and 39 patients started chemotherapy in the A-, B- and C-arm, respectively. Two patients did not receive any treatment in the study due to diagnosis of distant metastases on the day of randomisation in one patient and one withdraw consent.
In total, 305 cycles were given in the A arm (dose-dense and tailored), 315 in the B-arm (dose-dense, fixed doses) and 222 in the C-arm (TAC q3w).
In the dose dense arms six (14%) and four (10%) patients in arm A and B respectively received less than eight cycles. The corresponding figure for the TAC arm (arm C) was four (10%) patients.
Delayed courses. Accepting a 20% delay per cycle, i.e. a treatment interval of more than 16 days in arm A and B and more than 25 days in arm C, 24% of the patients in arm A, 21% in arm B and 3% in arm C had delays due to side-effects (Fisher´s exact test, p = 0.042). There were also delays due to other reasons in seven patients. Of the 24 events in arm A and B, 11 occurred before the amendment (11/124 = 9%) and 13 after the amendment (13/496 = 3%) (p = 0.003).
Given dose. In the majority of the patients in the A arm dose escalation was possible in cycle 2–4 (EC) and 6–8 (docetaxel) according to the predefined criteria and clinical assessment (, ).
Figure 1. Dose-intensity per cycle in the dose-dense and tailored arm (arm A). For dose-levels (steps) see .
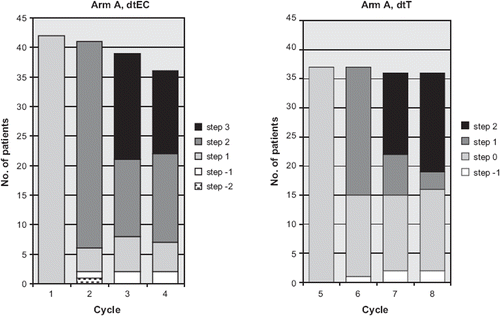
The mean cumulative dose of epirubicin per patient was 381 mg/m2 (arm A) and 357 mg/m2 (arm B) (p< 0.001, A vs. B) while the doxorubicin dose was 283 mg/m2 in the TAC arm. The mean cumulative cyclophosphamide doses per patient were 3118, 2395 and 2838 mg/m2 (p < 0.001), and for docetaxel the corresponding figures were 322, 276 and 416 mg/m2 (p < 0.001) in arm A, B and C respectively. Comparisons between the three arms were made using Kruskal-Wallis rank test.
Safety
Grade 3 and 4 adverse events. There were no toxicity related deaths in the study. One grade 4 (fatigue, arm A; cycle 5) and 134 grade 3 adverse events were reported in the study ( and ).
Table IIIa. Grade 3 adverse events per chemotherapy cycle in arm A, B and C respectively. The events in Arm A and B are further subdivided in the dose-dense epirubicin-cyclophosphamide part (dEC) and dose-dense docetaxel (dT) part.
Table IIIb. Grade 1, 2 and 3 adverse events per patient in cycle in arm A, B and C respectively.
Ten patients (24%) in the A arm experienced grade 3 infections/febrile neutropenia compared to two (5%) and six (15%) patients in the B- and C-arm respectively (Fisher´s exact test, p = 0.044). If grade 3 infections with normal neutrophil count (eight events in seven patients) were excluded the values were 15% (Arm A), 0% (arm B) and 15% (arm C).
Laboratory toxicity was seen for haemoglobin (grade 3, arm A and C, one patient each, none grade 4), white blood count (grade 3 and/or 4: arm A 34 patients, arm B 17 patients, arm C 34 patients), neutrophils (grade 3 and/or 4: arm A 34 patients, arm B 20 patients, arm C 35 patients), platelets (grade 3 and/or 4: arm A one patient, arm C two patients), and ALAT (grade 3: arm C one patient, none grade 4). There were no grade 3 or 4 toxicities for ASAT, ALP or bilirubin.
Hand-foot-skin reactions. An amendment was introduced after 124 cycles in the A and B arm due to six grade 3 hand-foot-skin reactions (four in arm A, two in arm B) during the docetaxel part (4.8%). After the addition of one week extra pause between the EC and T part, 496 cycles were given with two reported grade 3 hand-foot-skin reaction in the A arm (one in the EC-part and one in the T part) (0.4%).
Hospitalisation. There were 50 hospitalisation events (5.9% of all 842 cycles) in 36 patients in the study. Forty-three events in 32 patients were due to side-effects, while seven events in seven patients were due to other reasons. Twenty-nine percent (12 per arm) in the A and B arm, and 20% (eight patients) were hospitalised because of side-effects (Fisher´s exact test, p = 0.58). The main reasons for hospitalisation were fever/febrile neutropenia/infection (18 events), nausea (eight events, whereof four in the same patient), diarrhoea (four events), thrombosis (two events) and fatigue (two events).
Follow-up. One patient in the A-arm has been diagnosed with a GIST-tumour of the jejunum more than two years after completion of chemotherapy and a relation to given breast cancer treatment was regarded as improbable. No other secondary malignancies have been reported in the study.
Discussion
The SBG 2004-1 study is the first study of a both dose-dense and tailored taxane-containing adjuvant regimen. The study was planned as a randomised phase II study to evaluate the safety and feasibility of an anthracycline and docetaxel-containing regimen combining dose-density and tailoring (dtEC/dtT). The other arms were the same regimen with fixed doses (dEC/dT) and TAC, which served as a standard arm. Criteria concerning delayed chemotherapy cycles, grade 3 infections/febrile neutropenia and requirement of hospitalisation were predefined to serve as guidance whether to continue into a phase III study.
Regarding delayed treatments, the dose-dense arms did not fulfil this criterion (24% arm A, 21% arm B). Since the C arm consisted of only six cycles versus eight in the A and B arm, there were more possibilities for an event in the latter arms. If calculated in event per cycle, 4%, 4% and 0.9% of the cycles in the A, B and C arm respectively were delayed and the incidence in arm A and B lower after the amendment (9% versus 3% of the cycles, p = 0.003).
With regard to grade 4 infection /febrile neutropenia, all arms were feasible. However 23% in the A arm experienced a grade III infection /febrile neutropenia and did thus not fulfil the criterion of ≤20% infections. If grade 3 infections with normal ANC were excluded, the value was 15%.
There was also a criteria stipulating ≤20% of the patients requiring hospitalisation due to administered therapy. This was fulfilled in the C arm (20%) while 29% in the A and B arm were hospitalised, at least once, due to side-effects. If calculating events in relation to the total number of possible events (cycles) the figure was 6% in the A arm and 4% in the B- and C arm.
Apart from infections, fatigue and muscular-skeletal pain were the most common grade 3 toxicities (18 % and 14% in the A arm). In the ongoing phase III part of the study it is not recommended to escalate further if grade 2 fatigue or more, occurred during the previous cycle.
Several previous publications have reported non-feasibility of dose-dense docetaxel mainly due to hand-foot-skin-toxicity but also severe problems with nail disorders and peripheral neuropathy [Citation14–16]. These findings could not be confirmed in our study after the amendment of adding an extra week between EC and T. The initial dose of docetaxel was lower in our study compared to earlier studies (75 mg/m2 vs. 100 mg/m2) but 17 of the 42 patients in the A arm could in subsequent cycles be escalated to 100 mg docetaxel/m2 ().
There are also some data supporting that the sequence of taxanes and anthracyclines could affect toxicity (skin, haematological) and possibly also efficacy [Citation17]. Further adjuvant /neoadjuvant studies evaluating dose-dense regimens using taxanes first would be valuable.
The concept of tailoring of oncological therapies has recently gained some interest. In several retrospective studies it has been observed that patients receiving chemotherapy without haematological toxicity, have a worse outcome [Citation10,Citation18–21]. The same principle has also been observed for patients with non-small cell lung cancer and pancreatic cancer, respectively, treated with the epidermal growth factor tyrosine kinase inhibitor erlotinib [Citation22]. Patients with skin toxicity had better outcome [Citation22]. The monoclonal antibody cetuximab was studied in patients with colo-rectal cancer in a randomised study resulting in better response for those who received escalated cetuximab doses until skin toxicity [Citation23]. Finally, in a retrospective analysis of the ATAC study; patients receiving either anastrozole or tamoxifen in standard doses were doing worse if they did not experience side-effects likely related to oestrogen deprivation [Citation24]. One may speculate that the reasons for the above described relationships may be due to similar drug targets both in the normal tissue and in the tumour tissue respectively. The common theme for these observations are that patients have individual drug tolerances-metabolisms, not compensated by conventional oncological dosing strategies resulting in both under- and overdosing of the patients. The tailored dosing concept tested in the present study was aiming at compensating for underdosage.
The original intention of our study was to use the FEC regimen for the first four dose-dense cycles. However, after a publication reporting severe pleural and pericardial effusions on this regimen, the 5-fluorouracil was omitted [Citation25]. There was no pleural or pericardial adverse event in the present study.
Conclusion
The pre-stipulated criteria regarding toxicity were not fulfilled by the dose-dense and tailored arm, these criteria were however set up only as guidance and may have been too strict.
With some minor changes in the dose-dense and tailored arm, the concept of dose-dense and tailored EC/T is now being further explored as the experimental arm in a randomised phase III study (PANTHER, EudraCT number 2007-002061-12 and GovTrials number NCT00798070) in collaboration with the Austrian Breast and Colorectal Study Group (ABCSG) and German Breast Group (GBG) with more than 1400 randomised patients (September 2010).
Acknowledgements
This study was supported by grants from the Swedish Cancer Society, the Stockholm Cancer Society, the King Gustaf V Jubilee Fund, the Karolinska Institutet Research Funds, the Swedish Research Council, the Linné Grant and the Stockholm County Council, BRECT consortium and Märit and Hans Rausing's initiative against breast cancer together with research supports and grants from Sanofi Aventis, Chugai and Amgen.
Declaration of interest: Professor Jonas Bergh has stated the following: Research support for pharmacogenomics the last three years from Merck, USA, Orphan and Pfizer, USA. Advisory Boards the last three years for Affibodies, Abraxis Oncology, Amgen, Astra Zeneca, BMS, GSK, Bayer, ONYX/Bayer, Pfizer, Roche, Sanofi-Aventis. Educational activities/chairman etc with honoraria the last three years for Astra Zeneca, Novartis, Pfizer, Roche, Sanofi-Aventis. Research support for SBG 2004-1 from Amgen, Chugai, Sanofi-Aventis. The other authors have no conflicts of interest.
References
- Clarke M, Coates AS, Darby SC, Davies C, Gelber RD, Godwin J, . Adjuvant chemotherapy in oestrogen-receptor-poor breast cancer: Patient-level meta-analysis of randomised trials. Lancet 2008;371(9606):29–40.
- Polychemotherapy for early breast cancer: An overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 1998;352(9132):930–42.
- Levine MN, Pritchard KI, Bramwell VH, Shepherd LE, Tu D, Paul N. Randomized trial comparing cyclophosphamide, epirubicin, and fluorouracil with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node-positive breast cancer: Update of National Cancer Institute of Canada Clinical Trials Group Trial MA5. J Clin Oncol 2005;23:5166–70.
- Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla JP, Weaver C, . Adjuvant docetaxel for node-positive breast cancer. N Engl J Med 2005;352:2302–13.
- Roche H, Fumoleau P, Spielmann M, Canon JL, Delozier T, Serin D, . Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J Clin Oncol 2006;24: 5664–71.
- Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, . Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol 2006;24:5381–7.
- Ferguson T, Wilcken N, Vagg R, Ghersi D, Nowak AK. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst Rev 2007(4):CD004421.
- Martin M, Rodriguez-Lescure A, Ruiz A, Alba E, Calvo L, Ruiz-Borrego M, . Randomized phase 3 trial of fluorouracil, epirubicin, and cyclophosphamide alone or followed by Paclitaxel for early breast cancer. J Natl Cancer Inst 2008;100:805–14.
- Sandstrom M, Freijs A, Larsson R, Nygren P, Fjallskog ML, Bergh J, . Lack of relationship between systemic exposure for the component drug of the fluorouracil, epirubicin, and 4-hydroxycyclophosphamide regimen in breast cancer patients. J Clin Oncol 1996;14:1581–8.
- Wilking N, Lidbrink E, Wiklund T, Erikstein B, Lindman H, Malmstrom P, . Long-term follow-up of the SBG 9401 study comparing tailored FEC-based therapy versus marrow-supported high-dose therapy. Ann Oncol 2007;18:694–700.
- Bonnefoi HR, Bogaerts J, Piccart M, Mauriac L, Fumoleau P, Jassem J, . Phase III trial (EORTC 10994/BIG 00-01) assessing the value of p53 using a functional assay to predict sensitivity to a taxane versus nontaxane primary chemotherapy in breast cancer: Final analysis. 2010 ASCO Annual Meeting; 2010. J Clin Oncol 28:18s, 2010 (suppl; abstr LBA503); 2010.
- Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, . Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003;21:1431–9.
- Moebus V, Jackisch C, Lueck HJ, du Bois A, Thomssen C, Kurbacher C, . Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: Mature results of an AGO phase III study. J Clin Oncol 2010;28:2874–80.
- Piedbois P, Serin D, Priou F, Laplaige P, Greget S, Angellier E, . Dose-dense adjuvant chemotherapy in node-positive breast cancer: Docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Ann Oncol 2007;18:52–7.
- Di Leo A, Crown J, Nogaret JM, Duffy K, Bartholomeus S, Dolci S, . A feasibility study evaluating docetaxel-based sequential and combination regimens in the adjuvant therapy of node-positive breast cancer. Ann Oncol 2000;11:169–75.
- Schwartz J, Domchek SM, Hwang WT, Fox K. Evaluation of anemia, neutropenia and skin toxicities in standard or dose-dense doxorubicin/cyclophosphamide (AC)-paclitaxel or docetaxel adjuvant chemotherapy in breast cancer. Ann Oncol 2005;16:247–52.
- Wildiers H, Forceville K, Paridaens R, Joensuu H. Taxanes and anthracyclines in early breast cancer: Which first? Lancet Oncol 2010;11:219–20.
- Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB, . Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. J Natl Cancer Inst 1998;90:1205–11.
- Cameron DA, Massie C, Kerr G, Leonard RC. Moderate neutropenia with adjuvant CMF confers improved survival in early breast cancer. Br J Cancer 2003;89:1837–42.
- Poikonen P, Saarto T, Lundin J, Joensuu H, Blomqvist C. Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br J Cancer 1999;80:1763–6.
- Saarto T, Blomqvist C, Rissanen P, Auvinen A, Elomaa I. Haematological toxicity: A marker of adjuvant chemotherapy efficacy in stage II and III breast cancer. Br J Cancer 1997;75:301–5.
- Wacker B, Nagrani T, Weinberg J, Witt K, Clark G, Cagnoni PJ. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 2007;13:3913–21.
- Tejpar S, Peeters M, Humblet Y, Vermorken JB, De Hertogh G, De Roock W, . Relationship of efficacy with KRAS status (wild type versus mutant) in patients with irinotecan-refractory metastatic colorectal cancer (mCRC), treated with irinotecan (q2w) and escalating doses of cetuximab (q1w): The EVEREST experience (preliminary data). In: ASCO; 2008: JCO; 2008. p. Abstract 4001.
- Cuzick J, Sestak I, Cella D, Fallowfield L. Treatment-emergent endocrine symptoms and the risk of breast cancer recurrence: A retrospective analysis of the ATAC trial. Lancet Oncol 2008;9:1143–8.
- Dang CT, D'Andrea GM, Moynahan ME, Dickler MN, Seidman AD, Fornier M, . Phase II study of feasibility of dose-dense FEC followed by alternating weekly taxanes in high-risk, four or more node-positive breast cancer. Clin Cancer Res 2004;10:5754–61.