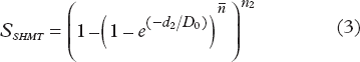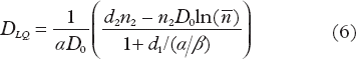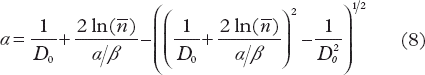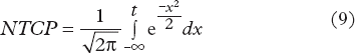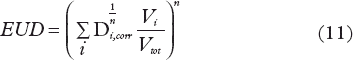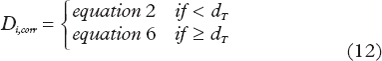Abstract
Background. In SBRT of lung tumours no established relationship between dose-volume parameters and the incidence of lung toxicity is found. The aim of this study is to compare the LQ model and the universal survival curve (USC) to calculate biologically equivalent doses in SBRT to see if this will improve knowledge on this relationship. Material and methods. Toxicity data on radiation pneumonitis grade 2 or more (RP2+) from 57 patients were used, 10.5% were diagnosed with RP2+. The lung DVHs were corrected for fractionation (LQ and USC) and analysed with the Lyman- Kutcher-Burman (LKB) model. In the LQ-correction α/β = 3 Gy was used and the USC parameters used were: α/β = 3 Gy, D0 = 1.0 Gy, ![]() = 10, α = 0.206 Gy−1 and dT = 5.8 Gy. In order to understand the relative contribution of different dose levels to the calculated NTCP the concept of fractional NTCP was used. This might give an insight to the questions of whether “high doses to small volumes” or “low doses to large volumes” are most important for lung toxicity. Results and Discussion. NTCP analysis with the LKB-model using parameters m = 0.4, D50 = 30 Gy resulted for the volume dependence parameter (n) with LQ correction n = 0.87 and with USC correction n = 0.71. Using parameters m = 0.3, D50 = 20 Gy n = 0.93 with LQ correction and n = 0.83 with USC correction. In SBRT of lung tumours, NTCP modelling of lung toxicity comparing models (LQ,USC) for fractionation correction, shows that low dose contribute less and high dose more to the NTCP when using the USC-model. Comparing NTCP modelling of SBRT data and data from breast cancer, lung cancer and whole lung irradiation implies that the response of the lung is treatment specific. More data are however needed in order to have a more reliable modelling.
= 10, α = 0.206 Gy−1 and dT = 5.8 Gy. In order to understand the relative contribution of different dose levels to the calculated NTCP the concept of fractional NTCP was used. This might give an insight to the questions of whether “high doses to small volumes” or “low doses to large volumes” are most important for lung toxicity. Results and Discussion. NTCP analysis with the LKB-model using parameters m = 0.4, D50 = 30 Gy resulted for the volume dependence parameter (n) with LQ correction n = 0.87 and with USC correction n = 0.71. Using parameters m = 0.3, D50 = 20 Gy n = 0.93 with LQ correction and n = 0.83 with USC correction. In SBRT of lung tumours, NTCP modelling of lung toxicity comparing models (LQ,USC) for fractionation correction, shows that low dose contribute less and high dose more to the NTCP when using the USC-model. Comparing NTCP modelling of SBRT data and data from breast cancer, lung cancer and whole lung irradiation implies that the response of the lung is treatment specific. More data are however needed in order to have a more reliable modelling.
Today more cancer patients will survive due to improved therapy regimens. In patients undergoing radiotherapy (RT) for tumours located in and nearby the thorax, irradiation of the healthy lung is unavoidable and subsequent radiation-induced pulmonary complications can be a serious problem, sometimes lethal, in patients with already reduced pulmonary capacity. The most frequent side effect, radiation pneumonitis (RP), characterised by dry cough, dyspnoea and fever, frequently occurs in patients treated for lung cancer (LC) and to a less extent in patients treated for breast cancer (BC).
With stereotactic body radiotherapy (SBRT) very high focal doses are applied to small volumes of lung tissue [Citation1,Citation2] making it possible to more than double the biological dose to the tumour with acceptable side-effects [Citation3]. In SBRT of peripheral lung tumours, several studies indicates that the incidence of clinical radiation pneumonitis (RP2+) is low (0–15%) [Citation4]. Volume of the CTV seems to the most important prognostic factor for development of RP. Furthermore, there also appears to be a weak correlation between prescribed dose and RP for relatively small CTVs [Citation5]. This is to some extent contradictory to clinical findings from conventional RT where an association between dose-volume parameters and RP has been demonstrated [Citation6]. The aim of this study is to compare different models (linear quadratic and the universal survival curve) to calculate biologically equivalent doses in SBRT to see if this will improve knowledge of how and if radiation induced lung reactions in SBRT are dependent on the irradiated volume, fractionation schedule and total dose. This will allow comparison of biological effects of treatments by stereotactic large dose per fraction schedules and allow tailoring treatment for each individual in daily routine practice keeping the incidence of pulmonary side-effects as low as possible.
In order to compare dose-volume parameters for different fractionation schedules and treatment modalities, doses must be converted to biologically equivalent doses for a standardised fractionation pattern, usually 2 Gy/fraction. The linear quadratic (LQ) model is the accepted model for conventionally fractionated radiotherapy (CFRT), but for extreme hypo-fractionation as used in SBRT it has been debated whether the LQ model is valid for fractionation correction [Citation7–11]. A hybridised model, the universal survival curve (USC), combining the LQ model in the low dose range and the Single Hit Multi-Target model (SHMT) in the high dose range proposed by Park et al. [Citation12] is one way to enhance the fit of the curve in a larger span in dose. In SBRT of lung-targets the typical situation is a large range of dose to the lungs. Today there is no decisive support from clinical data that promotes one over the other of the LQ and USC models for calculation of biologically equivalent doses for lung toxicity after SBRT.
Normal tissue complication probability (NTCP) modelling of toxicity may give an insight of the volume dependence for the radiation response mechanism. Commonly used NTCP models are based on three parameters describing the dose-response curve; a volume dependence parameter and two parameters describing the steepness and the dose where the probability of the selected response is 50%. As NTCP is dependent on the model used for fractionation correction of the dose-volume histogram we will study the impact on using the USC as well as the LQ.
Comparison between lung toxicity and dose-volume data from hypo-fractionation in SBRT and CFRT may give an insight of the radiation response when different parts of the lungs are irradiated, with a range of dose levels and lung volumes. In the supplement, toxicity and dose-volume data from the literature have been compiled and compared. The supplementary information is to be found online, at http//www.informahealthcare.com/(DOI: 10.3109/0284186X.2010.543695).
Material and methods
In this modelling study, based on data for RP2+ from a phase II trial of SBRT for stage I NSCLC, the fractionation correction was done both with the LQ and the USC models. NTCP modelling of lung toxicity after SBRT was performed with the Lyman-Kutcher-Burman (LKB) model. The purpose was to investigate the impact of the two models (LQ and USC) to calculate biologically equivalent doses on the volume dependence parameter of the LKB model.
Theory – survival curves and calculation of biological equivalent doses
The LQ model approximate clonogenic survival data with a linear quadratic formula where the survival S after n1 fractions with a dose of d1 Gy per fraction is given by
From Equation 1 it follows that n1 fractions given with d1 Gy per fraction is converted to a second fractionation scheme with n2 fractions given with d2 Gy per fraction by
For the SHMT model, the survival S after n2 fractions with a dose of d2 Gy per fraction is given by:
where D0 and ![]() describe respectively the slope and the extrapolation number respectively of the linear part in a plot of the logarithm of survival versus dose which the SHMT model approaches at high doses given by
describe respectively the slope and the extrapolation number respectively of the linear part in a plot of the logarithm of survival versus dose which the SHMT model approaches at high doses given by
In the USC model as suggested by Park et al. [Citation12], the LQ model smoothly transitions into the linear part of the SHMT model (linSHMT) at the transition dose dT given by
We will use the lower case nomenclature on dT as suggested by Borst et al. [Citation13] as the transition dose refers to the dose-per-fraction. From Equations 1 and 4 it follows that a total dose (n2d2) given in d2 Gy per fraction where d2 is greater than dT is converted to a total dose DLQ = n1d1 given in d1 Gy per fraction where d1 is less than dT by
Equation 6 may thus be used to convert hypofractionation schemes, with a dose per fraction larger than dT, to a fractionation scheme with a dose per fraction smaller than dT.
At dT the survival given by the LQ and linSHMT models are exactly the same:
For given values of D0 , ![]() and α/β, α is obtained from Equations 5 and 7 by
and α/β, α is obtained from Equations 5 and 7 by
The parameters D0, ![]() of the USC model were determined from constraints applied on dT and α. The parameter space of D0 and
of the USC model were determined from constraints applied on dT and α. The parameter space of D0 and ![]() that fulfilled the constraints (Equations 5 and 8) were calculated for an α/β value of 3 Gy ().
that fulfilled the constraints (Equations 5 and 8) were calculated for an α/β value of 3 Gy ().
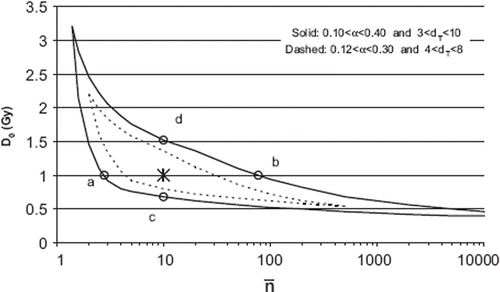
Figure 1. Parameter space of D0 and ![]()
Regarding constraints on α values, some data for different cell lines, mainly tumour cells, are given in the literature. For NSCLC Metha et al. [Citation14] and Park et al. [Citation12] reported an α value of 0.35 Gy−1. Lung tissue is proposed to have an α value of 0.19 Gy−1, as obtained from in vitro data from rats [Citation15]. In the determination of the USC model parameters D0, ![]() two different ranges of α values were evaluated: 0.10–0.40 Gy−1 and 0.12–0.30 Gy−1.
two different ranges of α values were evaluated: 0.10–0.40 Gy−1 and 0.12–0.30 Gy−1.
Regarding the transition dose dT Park et al. determined it to be 6.2 Gy for the NSCLC lines evaluated [Citation12]. Borst et al. found the best NTCP fit for RP at dT = 5 Gy from a clinical material of patients treated with SBRT [Citation13]. In lack of more detailed knowledge, two different ranges of dT values were evaluated: 3–10 Gy and 4–8 Gy. From the parameter space depicted in , D0 was chosen to be 1.0 Gy and ![]() to be 10 (marked by *). Given these values and α/β equal to 3 Gy, Equations 8 and 5 will give respectively α = 0.206 Gy−1 and dT = 5.8 Gy. The four points a-d in represents different parameter sets of D0 and
to be 10 (marked by *). Given these values and α/β equal to 3 Gy, Equations 8 and 5 will give respectively α = 0.206 Gy−1 and dT = 5.8 Gy. The four points a-d in represents different parameter sets of D0 and ![]() used as a sensitivity test of the α and dT values.
used as a sensitivity test of the α and dT values.
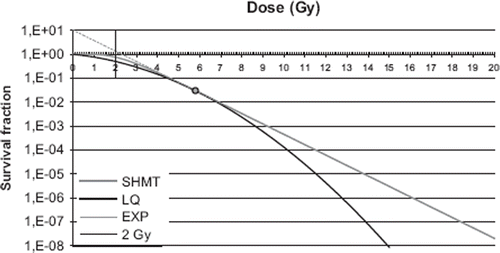
Survival curves calculated for the SHMT model (α/β = 3 Gy, D0 = 1.0 Gy, ![]() = 10 and α = 0.206 Gy−1) and the LQ model for α/β = 3, are shown in . The transition dose dT = 5.8 Gy is marked in the graph.
= 10 and α = 0.206 Gy−1) and the LQ model for α/β = 3, are shown in . The transition dose dT = 5.8 Gy is marked in the graph.
Figure 2. Calculated survival curves for the LQ model with α/β = 3, and α equal to 0.206 (black line), and for the SHMT model (grey line). The grey dashed line (EXP) shows the extrapolation of the linear portion of the SHMT target curve. The smooth transition from the LQ- to the linear portion of the SHMT model is at the dose dT = 5.8 Gy (indicated by the circle). The dose of 2 Gy, to which all DVH data were converted, is marked in the figure.
SBRT clinical data
A multi institutional phase II trial on medically inoperable patients with stage I NSCLC treated with SBRT was conducted from 2003 to 2005. Fifty-seven patients were included who were considered inoperable mainly due to chronic obstructive pulmonary disease or cardiovascular disease. The mean age of the patients was 74.3 year (range 63–82 years). The patients were treated with SBRT with 15 Gy × 3 prescribed to the 67% isodose at the periphery of the PTV, resulting in a central dose of about 22 Gy × 3. The incidence of RP2+ after a median follow-up of 23 months, according to the NCI-CTC v 2.0 toxicity grading system, was 10.5% [Citation16].
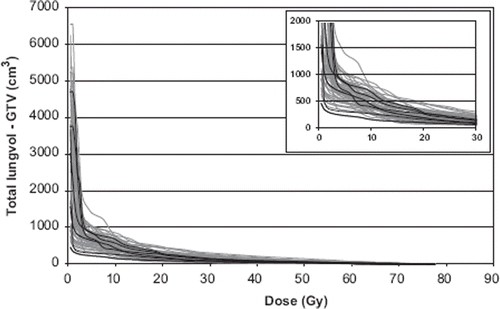
The mean dose and mean volume of the GTV were 65 Gy (uncorrected dose) and 17 cm3, respectively. The mean dose and mean volume of the PTV were 61 Gy (uncorrected dose) and 72 cm3, respectively. The lung is here defined as a paired organ and doses and volumes are reported for the total lung volume (both lungs) minus the GTV. The mean dose and the mean volume of the total lung-GTV were 4.3 Gy (uncorrected dose) and 2926 cm3, respectively. Patients with RP2+ had a mean dose of 4.1 Gy and a mean volume of 2699 cm3. Dose volume histograms (DVHs) for patients are presented in . Six patients with RP2+ are indicated in the graph.
Figure 3. Dose-volume-histograms (DVHs) of the total lung volume minus GTV in cm3 for the 57 SBRT patients. Six patients with RP2+ are marked with black lines. An enlargement for better visualization is inserted.
At the initiation of the study (2003) the dose calculation amongst participating institutions was based on the pencil beam (PB) algorithm. This algorithm was used for all patients on TMS-Helax (v6.1b) by Nucletron or Eclipse by Varian. Correction for inhomogeneities was used. The PB algorithm is known to overestimate the dose to the lung volume between the GTV and PTV [Citation17]. In the fractionation correction of the DVHs all bins (0.5 Gy) were corrected to 2 Gy/fraction.
NTCP modelling of SBRT data
In the present analysis, the parameter n of the LKB model was determined for an interval of values of m and D50. Individual DVH's were used, and n was determined so that the mean NTCP of the calculated NTCPs from the Nordic phase II trial [Citation16] was 10.5%. The reason for this methodology, instead of a maximum-likelihood fit of the individual patient data, for determination of all three parameters of the LKB model was the low number (6) of events of RP2+.
NTCP was defined by the Lyman-Kutcher- Burman (LKB) model [Citation18,Citation19]
where
and the equivalent uniform dose (EUD) was defined by
The doses Di were corrected to 2 Gy/fraction by the two described models for calculating biological equivalent doses: LQ, Equation 2 with an α/β = 3 Gy and USC, Equations 2 and 6 in 0.5 Gy steps for the complete DVH. The USC uses the LQ model up to the transition dose dT. For higher dose SHMT correction for fractionation was used.
In the analysis, the volume parameter n was a free modelling-parameter while the LKB parameters m and D50 were analysed in an interval 0.3 to 0.4 (m) and between 20 to 30 Gy (D50). The volume parameter n was determined in a fitting procedure of NTCP to the incidence of RP2+ of 10.5%. The sensitivity of the parameter n was investigated by changing the incidence to 5% and 15% compared to the real clinical incidence of 10.5%.
Finally, NTCP was also calculated with the parameter values obtained from CFRT; n = 0.9, m = 0.4 and D50 = 30 Gy [Citation20,Citation21] with correction for fractionation by the LQ model with α/β = 3 Gy.
Fractional NTCP
The LQ model predicts a lower survival compared to the USC model at high doses. This would mean that high-dose volumes would contribute more to the calculated NTCP if the doses are converted to 2 Gy/fraction with LQ compared to USC. However, another important factor that may counter-balance this is the volume-dependence factor n in the NTCP calculation. In order to understand the fractional contribution to the calculated NTCP from the different dose-bins the concept of fractional NTCP was used for both LQ- and USC-corrected DVHs [Citation22]. This gives an interpretation of the relative contribution of different dose levels to the calculated NTCP, which may give an insight to the questions of whether “high doses to small volumes” or “low doses to large volumes” are most important for lung toxicity.
In this work we define the fractional NTCPfract as the incremental growth of calculated NTCP after each dose-volume-histogram bin normalised to the calculated NTCP for respectively model studied. Or formally expressed NTCPfract = [1-NTCP(Dx)/NTCP(Dx = 0)], where Dx is the dose lower than a cut-off level which is not taken into account for in the calculation of NTCP.
NTCPfract was calculated from the DVH of a representative patient with both USC corrected DVH data with NTCP parameters n = 0.71, m = 0.4, D50 = 30 Gy and LQ corrected DVH data and NTCP parameters n = 0.87, m = 0.4, D50 = 30 Gy.
SBRT clinical data from literature
In this work a review of data on lung toxicity and dose/volume characteristics from SBRT of lung tumours was performed [Citation4,Citation16,Citation23–32]. The way that doses are reported varies greatly which makes a comparison between different studies to some extent difficult. In the present review of SBRT data, mean value of absorbed dose to lung (total lung or ipsilateral lung volume) or in some cases only V20 were reported. Summary of published SBRT data where reported. From the dose data we reconstructed the treatments and calculated DVH. The reconstructions were done either using our DVH-data that matched the published data (assuming that similar SBRT techniques generates the same shape of DVH's) or we planned a treatment with the published techniques aiming to achieve the same dose parameters as in the published study. Mean lung dose (MLD) was calculated using LQ corrected dose data and n = 1. Equivalent uniform dose (EUD) was calculated using USC corrected data and n = 0.71 ().
Results
The n values for the LQ and the USC fractionation correction, were determined for m = 0.4 or 0.3 and D50 = 30 Gy or 20 Gy (). The parameters chosen in the USC model, D0 = 1 and ![]() = 10, is indicated by the star (*) in .
= 10, is indicated by the star (*) in .
Table I. Values of the parameter n from the NTCP modelling.
Table II. Summary of published SBRT data. Incidences of RP2+ and estimated EUD.
The sensitivity of n (for m = 0.4 and D50 = 30 Gy) with USC parameters, D0 and ![]() , were analysed for the parameter-values at the four points a-d in , resulting in the following values of n; 0.55, 0.77, 0.62 and 0.74, respectively.
, were analysed for the parameter-values at the four points a-d in , resulting in the following values of n; 0.55, 0.77, 0.62 and 0.74, respectively.
The sensitivity of n (for m = 0.4 and D50 = 30 Gy) with the incidence using the USC fractionation correction, was evaluated. For an incidence of RP2+ of 5% or 15% instead of 10.5% () the results were n = 0.89 and n = 0.65, respectively.
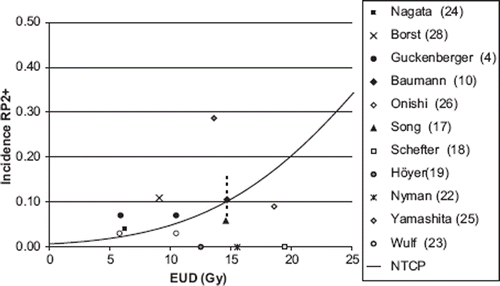
Figure 4. Incidence of RP2+ as a function of equivalent uniform dose (EUD) calculated with n = 0.71. The line shows NTCP calculated with parameters m = 0.30, D50 = 30 Gy. The value of the parameter n was determined from the data point EUD = 15 Gy and incidence of RP2+ = 10.5% (Baumann et al. 2008). As a sensitivity test of n, the incidences 5% (0.05) and 15% (0.15) at 15 Gy, were modelled (dotted line). References in .
Using LKB parameter values obtained from CFRT (n = 0.9, m = 0.37, D50 = 30 Gy) [Citation33] and the LQ model for fractionation correction the NTCP became 6.2% which underestimates the incidence of RP2+.
The NTCP modelling resulted in a volume parameter n for the LKB generally less than unity. As a consequence, MLD might not be a relevant parameter for the prediction of lung toxicity in SBRT. The incidence of RP2+, from the literature review, is consequently presented as a function of EUD, shown in and . The EUD values were calculated with n = 0.71. describes the assumptions done to calculate EUD for each data point. For comparison incidence data are presented as a function of MLD in the supplement, found online at http//www.informahealthcare.com/(DOI: 10.3109/0284186X.2010.543695).
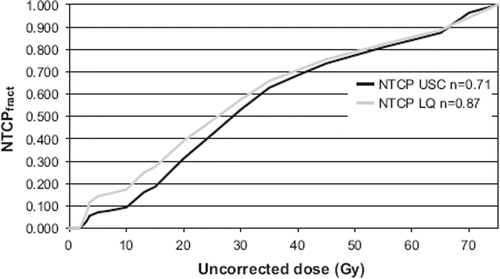
shows the NTCPfract with correction for fractionation based on either the USC (n = 0.71, m = 0.4, D50 = 30 Gy) or the LQ model (n = 0.87, m = 0.4, D50 = 30 Gy). At low doses there is a higher impact on the NTCP corrected with the LQ model (grey line) than with the USC model (black line), since the function NTCPfract grows faster for the LQ-model. As a result, the low dose is less important for the calculated NTCP using the USC model for fractionation correction while the higher doses have a higher impact. This is explained by the lower value of the n parameter for the USC correction compared to the LQ correction ().
Figure 5. Fractional NTCPfract calculated with DVH-data corrected with USC and LQ (α/β = 3) as a function of cut-off dose for a representative patient. The plot illustrates the cumulative contribution to the NTCP. With the USC correction the low doses have less impact on NTCP compared to what is seen with the LQ correction.
Discussion
In CFRT, a correlation has been established between DVH parameters and clinically relevant lung toxicity. This is, however, generally not the case in SBRT of targets in the lungs, with a few exceptions [Citation32,Citation34], Borst et al. [Citation7] found a significant dose-response relationship for RP after SBRT, but analysing literature data presented in and of RP2+ as a function of EUD, there is still no clear correlation between dose and RP. Uncertainties in calculating EUD and clinically scoring RP2+ might hide a correlation (see Discussion).
Survival curves and calculation of biological equivalent doses
There is some support for the USC model at high doses from in vitro data. Park et al. determined the dose-response curve for a survival fraction as low as 10−7 for H460 NSCLC cell lines by [Citation12]. However, there is a lack of in vivo data concerning lung toxicity for patients treated with extreme hypo-fractionation to promote or exclude any of the two models for fractionation correction [Citation13].
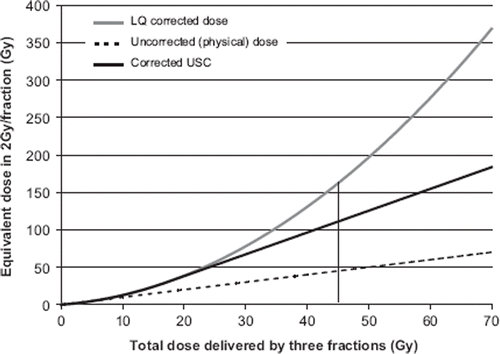
To visualise the different fractionation corrections for SBRT, shows the equivalent dose delivered in 2 Gy per fraction as a function of the total dose delivered in three fractions. The parameter values given in methods were used in the calculations. The uncorrected dose of 45 Gy given in 3 fractions is marked, since it was the prescribed dose at the periphery of the PTV in the clinical SBRT data set used here. The corrected doses at this dose level with the different models are for LQ equal to 162 Gy and for USC equal to 111 Gy.
Figure 6. The total uncorrected dose given in three fractions as a function of the dose given in 2 Gy per fraction (EQD2) using different fractionation correction models (LQ, uncorrected and USC). The dose level of 15 Gy × 3, used in the clinical data set is marked in the figure. Note, this refers to lung and not to tumour tissue.
NTCP modelling and EUD calculation
Regarding the sensitivity of USC parameters, D0 and ![]() , it can be concluded that even ‘extreme’ values of α and dT (a to d in ) still give D0 and
, it can be concluded that even ‘extreme’ values of α and dT (a to d in ) still give D0 and ![]() values that result in low values of n for the USC model compared to the LQ model. With the selected parameter values (D0 = 1.0 Gy,
values that result in low values of n for the USC model compared to the LQ model. With the selected parameter values (D0 = 1.0 Gy, ![]() = 10) n = 0.71 and at the points, a to d, the n value varies between 0.55 and 0.74 for the USC model compared to n = 0.87 for the LQ model.
= 10) n = 0.71 and at the points, a to d, the n value varies between 0.55 and 0.74 for the USC model compared to n = 0.87 for the LQ model.
In the NTCP modelling the parameter values used for D50 and m were respectively 20 Gy or 30 Gy and 0.3 or 0.4. Some arguments to support the studied interval of D50 and m are:
a) Modelling done for SBRT have suggested values for D50 20 Gy [Citation32,Citation34] and for CBRT 30 Gy are used [Citation35]. From Figure A1 in the appendix it can be seen that D50 is reasonably similar for whole lung irradiation and limited irradiation from breast/lung cancer RT. Thus, an even more focused/limited irradiation as in SBRT may be assumed to have a similar D50 value.
b) Looking at Figure A1 in the supplement (online) a clear difference of the slopes in the dose-response curves between whole lung irradiation (m = 0.2) and breast/lung cancer RT (m = 0.4) can be seen. Assuming that SBRT will have an even more shallow slope would imply that m has to be higher than 0.4. However, this is not consistent with the functional form of the NTCP model. A value much larger than 0.4 is mathematically unrealistic as the low dose region, which is the important region in this context, is not modelled correctly, with a non-zero NTCP value for a dose equal to zero.
The sensitivity of the parameter n, for m = 0.4 and D50 = 30 Gy, respectively, was evaluated by assuming an incidence of RP2+ to 5% and 15%. The result was accordingly, n = 0.84 and n = 0.62. It may be argued that the span of incidence of 5 to 15% as simulated here is consistent with the incidence data reported by other groups (with the exception of extreme values) as shown in .
In comparison, Borst et al. [Citation13] found values of D50 = 20.8 Gy and m = 0.45 assuming that n = 1, while Ricardi et al. reported D50 = 22.4 Gy [Citation34] in their modelling.
To explore whether the result of this study, was specific for the LKB-NTCP model, the relative seriality RS-NTCP model was used in a fitting procedure, with USC-corrected DVH-data obtaining s = 0.47, γ = 1, and D50 = 30 Gy. The result was consistent with what was seen with the LKB model, indicating a more serial architecture of the FSU. It should be noted that the RS model is based on different statistics where the seriality parameter s is high when a serial architecture is seen. Reported values of s for CFRT in BC and LC RT is of the order of 0.1 or less [Citation35].
Fractional NTCP
The question of whether “high doses to small volumes” or “low doses to large volumes” are most important for lung toxicity in SBRT still remains open. A consequence of the lower n value for the USC corrected data, compared to the LQ corrected, is that the volumes of lung tissue receiving a low dose will contribute less (relatively) to the calculated NTCP than volumes receiving high doses, as illustrated in . This finding in the NTCP modelling is not consistent to the experimental finding made by Semenko et al. [Citation36], where the lungs of rats were irradiated to different dose levels and volumes of lung tissue keeping the mean lung dose fixed. In that study the rats that were irradiated to a larger volume with lower doses experienced more toxicity than the ones that were irradiated to smaller volumes but with higher doses. Based on the lung-DVH data reported for the rats, NTCP modelling was performed. As a result a “super-parallel” architecture of the lung (n >1) has to be assumed in order to get a higher NTCP for the whole-lung irradiated rats, compared to those rats that were irradiated to smaller lung volumes. However, one of the methodological problems in the comparison between rat and the NTCP modelling of human data is that the present study deals with the probability of a specific end-point (RP2+), whereas in the rat study the breathing frequency was studied (a continuous parameter that reflects the deterioration in lung function). Furthermore the function of the lung differs between different mammals.
End point and DVH data for lung toxicity
The response of lung to radiation is a complex process and is usually divided into two phases; early and late [Citation37]. The acute phase develops one to six months after treatment, and late phase with established fibrosis develops and peaks at six months after therapy. It is varying in grade from asymptomatic to severe. In this work, RP2+ or higher was selected as end point, since RP2+ is a clinical meaningful condition for modelling that affects the patients quality of life. In addition, this endpoint led to a reasonable number of events for NTCP-modelling.
The end-point of RP is subjective and as such prone to be user dependent. Work has been done to diagnose RP by objective means with different clinical (lung physiology [Citation16,Citation26]) and radiological parameters (CT-scan [Citation4,Citation5]), but a correlation to clinical relevant side-effects has not yet been established.
The response of the lung after a high biologic effective dose given and concentrated to a small part of the lung, may be interpreted in terms of biological organisation, and stem from the complex anatomical structure of the lung with many different substructures such as the alveoli, the bronchial tree and also dependent on adjacent structures such as the pericardium. RP is not only caused by damage in the alveoli but also in the end bronchioles, the interstitial tissue and connecting capillaries. The alveoli are thought to work as structurally defined organs in parallel, which may have some correspondence to functional subunits of NTCP models. However, with very high fractional doses the surrounding tissues may translate the injuries to adjacent alveoli resulting in a response that could be interpreted as a more serial architecture of these functional subunits.
The lung is reported to have different sensitivity in different parts of the lung [Citation33,Citation38,Citation39]. This suggests that correlations between DVH parameters and toxicity as well as NTCP modelling will be specific for each target location and treatment technique within the thorax. The large span in toxicity data described () may be explained by this.
In SBRT, RP is likely to occur after the treatment, and might not be documented as an unexpected event. This means that lung toxicity is probably underreported in the literature, especially in retrospective studies. One author reports a surprisingly high incidence of severe lung toxicities [Citation30] as other have reported none [Citation24–27].
Another consideration is that confounding factors (smoking behaviour, lung function and chemotherapy prior to RT) influencing the response to radiation, are not always reported in SBRT studies.
Several sources of uncertainties exist in the calculation of the EUD values of . The calculations were based on reported dosimetric data of SBRT and in order to calculate EUD several assumptions were made, as described in . Furthermore, there are known issues about the accuracy of dose calculation algorithms in lung tissue [Citation40]. The pencil beam algorithm (PB) was earlier used by many centres but today more accurate algorithms based on 3-D scatter corrections are often used. The PB is known to overestimate the dose to the lung volume between the GTV and PTV and have been reported for SBRT cases [Citation17]. The impact of comparing dosimetric data in SBRT generated with different types of algorithms needs to be further investigated. However, in this study all participating centres used the PB algorithm.
Conclusion
In SBRT of lung tumours, NTCP modelling of lung toxicity RP2+ comparing the LQ and the USC models for fractionation correction, shows that low dose volumes contribute less and high dose volumes more to the NTCP when using the USC model. Comparing NTCP modelling of SBRT data and data from breast cancer, lung cancer and whole lung irradiation implies that the response of the lung is treatment specific. More clinical data are however needed in order to have a more reliable conclusion whether the NTCP model with USC correction for fractionation of DVH data improves the description of the response of the lung to hypo-fractionated regimes such as SBRT.
Review of lung toxicity data versus mean lung dose.
Download PDF (131.5 KB)Acknowledgements
Support of this study from the Swedish Cancer Society is gratefully acknowledged.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lax I, Blomgren H, Näslund I, Svanström R. Stereotactic radiotherapy of malignancies in the abdomen – methodological aspects. Acta Oncol 1994;33:677–83.
- Lax I, Larsson D, Näslund I. Extracranial stereotactic radiosurgery of localized targets. J Radiosurg 1998;1:135–48.
- Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator: Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861–70.
- Guckenberger M, Heilman K, Wulf J, Mueller G, Beckmann G, Flentje M. Pulmonary injury and tumor response after stereotactic body radiotherapy (SBRT): Results of a serial follow-up CT study. Radiother Oncol 2007;85:435–42.
- Kimura T, Matsuura K, Murakami Y, Hashimoto Y, Kenjo M, Kaneyasu Y, . CT appearance of radiation injury of the lung and clinical symptoms after stereotactic body radiation therapy (SBRT) for lung cancers: Are patients with pulmonary emphysema also candidates for SBRT for lung cancers? Int J Radiat Oncol Biol Phys 2006;66: 483–91.
- Rodrigues G, Lock M, D'Souza D, Yu E, Van Dyk J. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer – a systematic review. Radiother Oncol 2004;71:127–38.
- Borst GR, Shirato H, Nijkamp J, Onimaru R, Ishikawa M, Lebesque JV, Sonke J. Radiation pneumonitis for stereotactic irradiated lung cancer patients: Is the LQ model valid for high doses per fraction? Int J Radiat Oncol Biol Phys 2008; 72(1Suppl. 1):S68–69.
- Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol 2008;18:234–9.
- Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol 2008;18:240–3.
- Fowler JF. Linear quadratics is alive and well: In regard to Park et al. (Int J Radiat Oncol Biol Phys 2008;70:847–52). Int J Radiat Oncol Biol Phys 2008;72:957.
- Park C, Papiez L, Timmerman RD. In reply to Dr. Fowler and Dr. Kavanagh. Int J Radiat Oncol Biol Phys 2008; 72:958.
- Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:847–52.
- Borst GR, Ishikawa M, Nijkamp J, Hauptmann M, Shirato H, Bengua G, . Radiation pneumonitis after hypo-fractionated radiotherapy: Evaluation of the lq(l) model and different dose parameters. Int J Radiat Oncol Biol Phys 2010 Epub 2010 Mar 16.
- Mehta M, Scrimger R, Mackie R, Paliwal B, Chappell R, Fowler J. A new approach to dose escalation in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2001;49:23–33.
- Thames H, Hendry J. Fractionation in radiotherapy. Thames H, Hendry J. London: Taylor and Francis; 1987.
- Baumann P, Nyman J, Hoyer M, Gagliardi G, Lax I, Wennberg B, . Stereotactic body radiotherapy for medically inoperable patients with stage I non-small cell lung cancer – a first report of toxicity related to COPD/CVD in a prospective phase II study. Radiother Oncol 2008;88:359–67.
- Panettieri V, Wennberg B, Gagliardi G, Duch MA, Ginjaume M, Lax I. SBRT of lung tumours: Monte Carlo simulation with PENELOPE of dose distributions including respiratory motion and comparison with different treatment planning systems. Phys Med Biol 2007;52:4265–81.
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method. Int J Radiat Oncol Biol Phys 1989; 16:1623–30.
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl 1985;8:S13–19.
- Martel MK, Ten Haken RK, Hazuka MB. Dose-volume histogram and 3-D treatment planning evaluation of patients with pneumonitis. Int J Radiat Oncol Biol Phys 1994;28:575–81.
- Burman C, Kutcher GJ, Emami B. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991;21:123–35.
- Lax I, Karlsson B. Prediction of complications in Gamma-Knife radiosurgery of arteriovenous malformations. Acta Oncol 1996;35:49–55.
- Song D, Song DY, Benedict SH, Cardinale RM, Chung TD, Chang MG, . Stereotactic body radiation therapy of lung tumors: Preliminary experience using normal tissue complication probability-based dose limits. Am J Clin Oncol 2005; 28:591–6.
- Schefter TE, Kavanagh BD, Raben D, Kane M, Chen C, Stuhr K, . A phase I/II trial of stereotactic body radiation therapy (SBRT) for lung metastases: Initial report of dose escalation and early toxicity. Int J Radiat Oncol Biol Phys 2006;66(4 Suppl 1):S120–7.
- Hoyer M, Roed H, Traberg Hansen A, Ohlhuis L, Petersen J, Nellemann H. Prospective study on stereotactic radiotherapy of limited-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;66(4 Suppl 1):S128–35.
- Paludan M, Traberg Hansen A, Petersen J, Grau C, Hoyer M. Aggravation of dyspnea in stage I non-small cell lung cancer patients following stereotactic body radiotherapy: Is there a dose-volume dependency? Acta Oncol 2006;45:818–22.
- Nyman J, Johansson K-A, Hulten U. Stereotactic hypo-fractionated radiotherapy for stage I non-small cell lung cancer – Mature results for medically inoperable patients. Lung Cancer 2006;51:97–103.
- Wulf J, Haedinger U, Oppitz U, Thiele W, Mueller G, Flentje M. Stereotactic radiotherapy for primary lung cancer and pulmonary metastases: A noninvasive treatment approach in medically inoperable patients. Int J Radiat Oncol Biol Phys 2004;60:186–96.
- Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, . Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427–31.
- Yamashita H, Nakagawa K, Nakamura N, Koyanagi H, Tago M, Igaki H, . Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat Oncol 2007;2:21.
- Onishi H, Kuriyama K, Komiyama T, Tanaka S, Sano N, Marino K, . Clinical outcomes of stereotactic radiotherapy for stage I non-small cell lung cancer using a novel irradiation technique: Patient self-controlled breath-hold and beam switching using a combination of linear accelerator and CT scanner. Lung Cancer 2004;45:45–55.
- Borst GR, Ishikawa M, Nijkamp J, Hauptmann M, Shirato H, Onimaru R, . Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypo fractionated radiation therapy. Radiother Oncol 2009; 91:307–13.
- Seppenwoolde Y, De Jaeger K, Boersma LJ, Belderbos JSA, Lebesque JV. Regional differences in lung radiosensitivity after radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;60:748–58.
- Ricardi U, Filippi AR, Guarneri A, Giglioli FR, Mantovani C, Fiandra C, . Dosimetric predictors of radiation-induced lung injury in stereotactic body radiation therapy. Acta Oncol 2009;48:571–7.
- Seppenwoolde Y, Lebesque JV, de Jaeger K, Belderbos JSA, Boersma LJ, Schilstra C, . Comparing different NTCP models that predict the incidence of radiation pneumonitis. Int J Radiat Oncol Biol Phys 2003;55:724–35.
- Semenenko VA, Molthen RC, Li C, Morrow NV, Li R, Ghosh SN, . Irradiation of varying volumes of rat lung to same mean lung dose: A little to a lot or a lot to a little? Int J Radiat Oncol Biol Phys 2008;71:838–47.
- Oetzel D, Schraube P, Hensley F, Sroka-Perez G, Menke M, Flentje F. Estimation of pneumonitis risk in three-dimensional treatment planning using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys 1995;33: 455–60.
- Yorke ED, Jackson A, Rosenzweig KE, Merrick SA, Gabrys D, Venkatraman ES, . Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 2002;54: 329–39.
- Wennberg B, Gagliardi G, Sundbom L, Svane G, Lind P. Early response of lung in breast cancer irradiation: Radiologic density changes measured by CT and symptomatic radiation pneumonitis. Int J Radiat Oncol Biol Phys 2002;52:1196–206.
- De Jaeger K, Hoogeman MS, Engelsman M, Seppenwoolde Y, Damen E, Mijnheer B, . Incorporating an improved dose-calculation algorithm in conformal radiotherapy of lung cancer: Re-evaluation of dose in normal lung tissue. Radiother Oncol 2003;69:1–10.