Abstract
Background. The efficacy and safety of concurrent administration of irinotecan with the two monoclonal antibodies cetuximab and bevacizumab as fourth line therapy in heavily pretreated patients with metastatic colorectal cancer were evaluated. Patients and methods. Patients with metastatic colorectal cancer who had progressed on therapy with 5-FU, oxaliplatin and irinotecan in the first and second line setting and on the combination of irinotecan and cetuximab in third line setting independent of their KRAS mutation status, were treated with irinotecan and cetuximab combined with bevacizumab in a dosage of 5 mg/kg. All drugs were administered every second week. Results. From January 2007 to November 2008 27 patients were treated with cetuximab, irinotecan and bevacizumab. The triple-combination was well tolerated. Progression free survival (PFS) was 8.3 months and median overall survival (mOS) was 12.0 months. Two patients without KRAS mutation (7%) obtained a partial response and 17 (63%) had stable disease for at least two months. A retrospective KRAS mutation analysis revealed that there was a trend toward longer PFS and mOS in patients without KRAS mutations compared to patients with KRAS mutations with a PFS of 8.9 vs. 5.1 months and a mOS of 12.7 vs. 9.0 months. Conclusion. Bevacizumab is safe to add to irinotecan and cetuximab with a toxicity profile that seems to be similar to what would be expected from the agents alone. The results indicate that adding bevacizumab to irinotecan and cetuximab in a fourth line setting may induce a high rate of disease control in heavily pretreated patients with metastatic colorectal cancer.
Cetuximab is a human-murine chimeric monoclonal antibody inhibiting the epidermal growth factor receptor (EGFR) [Citation1]. In combination with irinotecan it is a well established treatment of patients with chemotherapy refractory metastatic colorectal cancer. Its effect was first established in 2004 in a study by Cunningham, the so-called “BOND-1 study” [Citation2]. This study showed that it was possible to overcome resistance to irinotecan by adding cetuximab. Originally cetuximab was administrated as a weekly infusion [Citation2–4], however cetuximab can also be administrated biweekly, probably without compromising efficacy [Citation5,Citation6]. Despite progressive disease to irinotecan and cetuximab in third line treatment, many patients maintain an excellent performance status, but so far no treatment has been proven effective in this setting and survival is short without further therapy. In two third line studies cetuximab [Citation3] and panitumumab [Citation7] was compared to best supportive care and in both studies they found a PFS of less than two months and a mOS of only about five months in the group without further therapy. Bevacizumab is a humanized monoclonal antibody directed against vascular endothelial growth factor A (VEGF-A) [Citation8]. In a small study by Saltz, the so-called “BOND-2 study” [Citation9], both bevacizumab and cetuximab were added to irinotecan. This study showed promising results with improved efficacy compared to the BOND-1 study.
Inspired by this study and by the possibility to overcome resistance to the combination of irinotecan and cetuximab we evaluated the safety and efficacy of adding bevacizumab to cetuximab and irinotecan in patients who had progressed on irinotecan and cetuximab. The patients were treated independent of their KRAS mutation status and of their EGFR receptor status.
Patients and methods
Patients with histological confirmed colorectal cancer and measurable disease by the Response Evaluation Criteria in Solid Tumors (RECIST) were included. All patients had progressed on therapy with 5-FU, oxaliplatin and irinotecan in first and second line and on irinotecan and cetuximab in third line therapy independent of their KRAS mutation status. Nearly 50% of the patients were still fit for further therapy, mainly with a performance status of 0 or 1. None of the patients had previously received therapy with bevacizumab.
The patients received cetuximab 500 mg/m2 infused within 60 minutes immediately followed by irinotecan 180 mg/m2 over 30 minutes every second week. In case of dose reduction in third line treatment, the reduced dose was maintained in the fourth line treatment where bevacizumab 5 mg/kg every second week was added. The patients were treated without knowledge of their KRAS mutation status. Pre-treatment included 100 mg prednisolone p.o., 2 mg clemastine i.v., ondansetrone 8 mg p.o. and 10 mg metoclopramide p.n. Treatment was continued until progression or unacceptable toxicity. Toxicity was prospectively evaluated according to NCI-CTCAE, version 3.0.
Tumor response was evaluated by a computed tomography scan of the thorax and abdomen every eight weeks according to the RECIST version 1.0.
Progression free survival was calculated from the first administration of cetuximab, irinotecan and bevacizumab to the first observation of disease progression or death from any cause. Median overall survival was calculated from the first administration of cetuximab, irinotecan and bevacizumab to death from any cause, with censuring at January 2010 which was the date our data was calculated. The Kaplan-Meier method was used to estimate the distribution of time to events for PFS and mOS. In 26 patients the patient primary biopsy were analyzed for seven mutations in K-RAS codon 12 and 13 using the DsX kit.
Results
Patient characteristics
Twenty-seven patients started treatment with cetuximab, irinotecan and bevacizumab from January 2007 to November 2008. All patients had progressed on prior therapy with 5-FU, oxaliplatin, irinotecan and cetuximab. None of the patients had received bevacizumab. The patient characteristics are outlined in . The median time from non-resectable disease to start of treatment was 31.5 months and the median time from last treatment with cetuximab and irinotecan to start of treatment was 2.4 months.
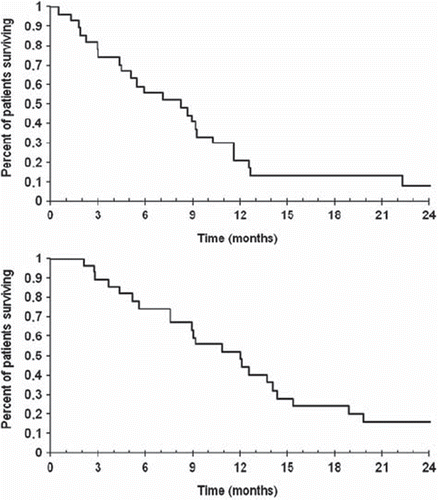
Table I. Kaplan-Meier curves of time to progression (median 8.3 months; 95% CI 5.1–9.3 months) and overall survival (median 12.0 months; 95% CI 8.9–14.1 months).
Toxicity
As seen in treatment was well tolerated with a low grade 3 or 4 toxicity included skin-toxicity in two patients (8%), diarrhea in one (4%), fatigue in one (4%) and neutropenia in one (4%). None of the patients developed grade 3 or 4 bleeding, thrombosis or hypertension.
Table II. Toxicity of treatment with biweekly cetuximab, irinotecan and bevacizumab.
Efficacy
The median PFS was 8.3 months (95% confidence interval (CI) 5–9 months) and the mOS was 12.0 months (95% CI 9–14 months) in the entire population (). Response rates were none complete response (0%), two partial response (7%), 17 stable disease (63%), for at least two months and five progressive disease (30%). After ending therapy 26 of the 27 patients had a K-RAS mutation determination. Patients with K-RAS wild-type did better than patients with K-RAS mutated-type, PFS 8.9 vs. 5.1 months and mOS 12.7 vs. 9.0 months. Both patients with PR were K-RAS wild-type ().
Table III. Efficacy data for patients receiving cetuximab, irinotecan and bevacizumab (CIB) as fourth line therapy.
Discussion
In the pivotal randomized phase II study by Cunningham [Citation2] cetuximab was added to irinotecan in patients who progressed on irinotecan. The study showed that in some patients it was possible to overcome resistance to irinotecan by adding cetuximab. In the explorative phase II study by Saltz [Citation9], both bevacizumab and cetuximab were added to irinotecan in patients who had progressed on irinotecan. Compared to data from the Cunningham study, the triple-combination showed promising results, raising the hypothesis that it might be possible to obtain further therapeutic gain by adding bevacizumab to irinotecan and cetuximab. Halama et al. [Citation10] had done a report of similar treatment with cetuximab, irinotecan and bevacizumab to five heavily pretreated patients and found response in four of five patients. In the first line setting however, other studies have questioned the benefit of concurrently bevacizumab and cetuximab or panitumumab. In the CAIRO-2 study [Citation11] cetuximab was added to bevacizumab, capecitabine and oxaliplatin and in the PACCE study [Citation12] panitumumab was added to FOLFOX + bevacizumab or FOLFIRI + bevacizumab. Both studies found that the combination of bevacizumab and EGFR inhibitors were detrimental. However both studies are first line treatment, while our series as well as both the series reported by Halama et al. as well as the BOND-2 study are all third or fourth line treatment. It would appear that monoclonal antibodies act different in patients that are chemotherapy naïve, compared to patients that are heavily pretreated. While the exact explanation for this is unknown, several hypotheses have been postulated. One possibility is that exposure to chemotherapy induces adaptive changes in the cancer cell that increases it's receptability to EGFR and VEGFR directed antibody therapy possibly influencing their signaling pathways. Another possibility may be that the selected population who is still in a good condition to receive third line treatment is the same selected population that benefit from concurrently bevacizumab and cetuximab therapy. Due to these reasons, concurrently treatment with cetuximab and bevacizumab might be a good treatment option in third or fourth line treatment, even if it is not the case in the first line setting. The low incidence of grade 3 or 4 toxicity found in this study is caused by the fact that we continued dose adjusted acceptable doses of irinotecan and cetuximab when adding bevacizumab.
In conclusion, we found that bevacizumab is safe to add to irinotecan and cetuximab in the fourth line setting, with a toxicity pattern that seems to be similar to what would be expected from the agents alone. Further, we found that adding bevacizumab induces a high rate of disease control at about 70% independent of KRAS mutation status with a PFS of 8.3 months. This is four times longer compared to best supportive care from historical data [Citation3,Citation7,Citation13,Citation14] and also leading to a high mOS of 12.0 months. This trial suggest that concurrently bevacizumab and EGFR inhibitors could be an option in heavily pretreated patients with colorectal cancer, but further trials are needed.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Brentjen R, Saltz L. Epidermal growth factor receptor blockade and treatment of solid tumor malignancies. De Vita JVT, Helmann S, Rosenberg SA, Progress in oncology. Boston, MA: Jones and Bartlett; 2002. 113–28.
- Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, . Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337–45.
- Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au H, . Cetuximab for the treatment of colorectal cancer. N Engl J Med 2007;357:2040–8.
- Pfeiffer P, Nielsen D, Yilmaz M, Iversen A, Vejlø C, Jensen BV. Cetuximab and irinotecan as third line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Acta Oncol 2007;46:697–701.
- Pfeiffer P, Nielsen D, Bjerregaard J, Qvortrup C, Yilmaz M, Jensen B. Biweekly cetuximab and irinotecan as third-line therapy in patients with advanced colorectal cancer after failure to irinotecan, oxaliplatin and 5-fluorouracil. Ann Oncol 2008;19:1141–5.
- Tabernero J, Pfeiffer P, Cervantes A. Administration of cetuximab every 2 weeks in the treatment of metastatic colorectal cancer: An effective, more convenient alternative to weekly administration? Oncologist 2008;13:113–9.
- Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, . Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658–64.
- Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr Rev 2004;25:581–611.
- Saltz LB, Lenz HJ, Kindler HL, Hochster HS, Wadler S, Hoff PM, . Randomized Phase II Trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: The BOND-2 Study. J Clin Oncol 2007;25:4557–61.
- Halama N, Herrmann C, Jaeger D, Hermann T. Treatment with cetuximab, bevacizumab and irinotecan in heavily pretreated patients with metastasized colorectal cancer. Anticancer Res 2008;28:4111–6.
- Tol J, Koopman CJ, Cats A, Cats A, Creemers GJ, Schrama JG, . A randomized phase III study on capecitabine, oxaliplatin and bevacizumab with or without cetuximab in first-line advanced colorectal cancer, the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG). An interim analysis of toxicity. Ann Oncol 2008;19:734–8.
- Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, . A randomized phase IIIB trial of chemotherapy, bevacizumab and panitumumab compared with chemotherapy and bevacizumab alone for metastasis colorectal cancer. J Clin Oncol 2009;5:672–80.
- Amado RG, Wolf M, Peeters M, Cutsem E, Siena S, Freeman DJ, . Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:1626–34.
- Karapetis CS, Khambata-Ford S, Jonker D, O´Callagan CJ, Tu D, Tebbutt NC, . K-RAS mutations and benefit from Cetuximab in advanced colorectal cancer. New Engl J Med 2008;359:1757–65.