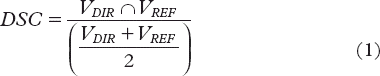Abstract
Background and purpose. Daily organ motion occurring during the course of radiotherapy in the pelvic region leads to uncertainties in the doses delivered to the tumour and the organs at risk. Motion patterns include both volume and shape changes, calling for deformable image registration (DIR), in approaches involving dose accumulation and adaptation. In this study, we tested the performance of a DIR application for contour propagation from the treatment planning computed tomography (pCT) to repeat cone-beam CTs (CBCTs) for a set of prostate cancer patients. Material and methods. The prostate, rectum and bladder were delineated in the pCT and in six to eight repeat CBCTs for each of five patients. The pCT contours were propagated onto the corresponding CBCT using the Multi-modality Image Registration and Segmentation application, resulting in 36 registrations. Prior to the DIR, a rigid registration was performed. The algorithm used for the DIR was based on a ‘demons’ algorithm and the performance of it was examined quantitatively using the Dice similarity coefficient (DSC) and qualitatively as visual slice-by-slice scoring by a radiation oncologist grading the deviations in shape and/or distance relative to the anatomy. Results. The average DSC (range) for the DIR over all scans and patients was 0.80 (0.65–0.87) for prostate, 0.77 (0.63–0.87) for rectum and 0.73 (0.34–0.91) for bladder, while the corresponding DSCs for the rigid registrations were 0.77 (0.65–0.86), 0.71 (0.55–0.82) and 0.64 (0.33–0.87). The percentage of propagated contours of good/acceptable quality was 45% for prostate; 20% for rectum and 33% for bladder. For the bladder, there was an association between the average DSC and the different scores of the qualitative evaluation. Conclusions. DIR improved the performance of pelvic organ contour propagation from the pCT to CBCTs as compared to rigid registration only. Still, a large fraction of the propagated rectum and bladder contours were unacceptable. The image quality of the CBCTs was sub-optimal and the usability of CBCTs for dose accumulation and adaptation purposes is therefore likely to benefit from improved image quality and improvements of the DIR algorithm.
Daily organ motion occurring throughout the course of radiotherapy (RT) for pelvic tumour sites leads to uncertainties in the doses delivered to both the tumour and the organs at risk (ORs) [Citation1,Citation2]. The introduction of volumetric image-guided radiotherapy (IGRT) using for instance on-line cone-beam computed tomography (CBCT) imaging on a daily basis has shown promising potential to reveal significant changes in patient anatomy relative to the original treatment plan [Citation3–5]. Better knowledge of the precise position of the mobile pelvic organs would improve the accuracy of treatment and eventually allow reduction of the safety margins around the tumour [Citation6,Citation7], enabling tumour dose escalation without increasing normal tissue doses [Citation6]. Clinically, IGRT methods are currently used for patient repositioning by rigidly aligning the images to the treatment planning CT (pCT). However, the internal anatomical changes are often extensive, including changes in both shape and size and might shift ORs towards the high-dose region [Citation8–10]. In order to predict normal tissue toxicity, knowledge of how the internal organ motion influences the dose distribution is central to obtain accurate dose/volume constraints [Citation5]. Previous studies have investigated normal tissue toxicity in relation to the internal organ motion by using population-based approaches, for example volume expansion, leading to significant associations between morbidity and dose-volume histogram (DVH) parameters for a broader range of dose levels [Citation11]. Aiming for patient-specific prediction of normal tissue toxicity, a dose distribution that reflects the dose actually delivered to the OR, including information of the complex organ motion is likely to be of considerable benefit [Citation5,Citation10,Citation12–14].
The rigid alignment between the pCT and the repeat CBCT scans used for daily IGRT assumes that organs experience only rotations and translations relative to one another. Rigid registration methods are therefore unable to account for the complex organ motion exhibited by key ORs in the pelvic region such as the rectum and bladder [Citation13,Citation15–17]. Deformable image registration (DIR) on the other hand is a method for tracking the complex organ motion on a voxel level [Citation12]. Ultimately, tracking also the dose distribution to each voxel would enable calculation of the treatment fraction dose to the tumour and the ORs [Citation5,Citation12,Citation16] and eventually also calculation of the accumulated dose to these organs [Citation5,Citation7,Citation12,Citation15,Citation16,Citation18,Citation19]. This is likely to generate a more representative dose distribution [Citation4,Citation5,Citation20] which is important for both adaptive RT (ART) [Citation5,Citation12,Citation15,Citation20,Citation21] and for prediction of normal tissue toxicity [Citation5], the latter presently being based on the information from the pCT only [e.g. 11,22]. However, both rectum and bladder are hollow organs, i.e. the surface is a reasonable approximation of the organ [Citation2,Citation15,Citation16]. DIR-based propagation of the delineated contours in the pCT onto CBCT scans therefore provides a method to assess the accuracy of the DIR [Citation13,Citation18,Citation23].
In this study, the performance of a commercial DIR algorithm has been evaluated in the pelvic region, for contour propagation of both tumour (prostate) and key ORs (rectum and bladder) from the pCT to repeat CBCT scans obtained throughout the treatment course. The accuracy of the algorithm was examined quantitatively using a commonly applied similarity measure and qualitatively as visual slice-by-slice inspection to investigate its potential for clinical introduction.
Material and methods
Image acquisition
Five patients treated for prostate cancer (PC) with intensity-modulated RT (IMRT) at Aarhus University Hospital in 2009 were included in this study. For each patient, the image sets consisted of the pCT and repeat CBCT scans (On-board-imager OBI, v.1.4, Varian Medical Systems, Palo Alto, CA, USA), evenly distributed throughout the treatment course. The patients were selected on the criteria of having at least five CBCT scans with sufficient cranio-caudal extension to ensure that both the prostate and the main ORs (rectum and bladder) were within the scan field-of-view (FOV). The included patients had between six to eight repeat CBCT scans each, resulting in 36 scans in total. The CBCT scans contained on average 55 slices (range: 53–58) reconstructed with 3 mm slice thickness, 512 × 512 pixels and 0.88 × 0.88 mm in-slice pixel resolution and were acquired with the OBI in a standard acquisition mode (125 kV, 80 mA, 13 ms and with 650 projections during 360°) covering a FOV diameter of 45 cm and a cranio-caudal extension of 16 cm. The pCT was obtained with a fan-beam helical CT scanner (Philips Mx8000 IDT 16, Philips Medical Systems, Eindhoven, The Netherlands) with 3 mm slice thickness, 512 × 512 pixels and an in-slice pixel resolution of 0.98 × 0.98 mm for a total of on average 98 slices (range: 85–111). The CBCT scans as well as the pCT were acquired in supine position. All patients had gold fiducial markers implanted in the prostate for daily set-up correction according to our clinical IGRT programme.
Organ definition
For this study specifically, a radiation oncologist (MH) outlined the prostate, the rectum and the bladder on the pCT within our treatment planning system (Eclipse, Varian Medical Systems). These organs were subsequently contoured on each CBCT scan by the same radiation oncologist for consistent delineation. The rectum was defined to extend from the recto-sigmoid junction in the cranial direction to the anal verge (or the last slide which included the ano-rectum) in the caudal direction, including both the wall and its contents; correspondingly the bladder included the organ wall and its contents. At our department all PC patients are given written and oral instructions to empty their bladder prior to each treatment session.
Image registration
For each registration, the contours in the pCT were retrospectively propagated onto each of the corresponding CBCT scans, in a systematic approach. To restrict influence from large deformations and to improve the speed and accuracy of the DIR the images were rigidly registered prior to the DIR [Citation4,Citation13,Citation15,Citation17]. All registrations were carried out in the Multi-modality Image Registration and Segmentation application (MIRS v.1.0, Varian Medical Systems, Palo Alto, CA, USA). The rigid registration was based on bony anatomy (200–3000 Hounsfield Units, HU) where the image covering the smaller FOV (typically the CBCT scan) determined the volume of interest (VOI).
The algorithm in the DIR application uses a ‘demons’ algorithm [Citation18], originally introduced by Thirion [Citation24]. The registration was based on intensity information using the HUs only [Citation3]. Organ changes in two images are managed by allowing one image to penetrate the boundaries of another [Citation17]. The algorithm is driven by demons that exert forces derived from the image referred to as the static image (typically the pCT) on the image containing the anatomical changes, i.e. the moving image, to match its intensity to that of the static image [Citation15]. This will aid the moving image to be brought in alignment voxel-by-voxel with the static image [Citation17,Citation24]. The forces in a demons algorithm are based on approximations and a crucial point is the regularisation of the forces [Citation4]. The DIR used in this study exploited a modified demons algorithm, which gradually increases the resolution [Citation17]. The algorithm also combined the forces derived from the static image with additional forces on the moving image to make the registration more efficient and to overcome large deformations [Citation3,Citation15,Citation17]. This algorithm has recently been described and its performance thoroughly evaluated on pCT-CT registrations in a study of PC patients from our group (denoted as algorithm ‘A’ in reference [Citation25]).
Evaluation
The contours obtained by propagating the pCT contours (prostate, rectum and bladder) onto each CBCT scan were examined both quantitatively and qualitatively with respect to the correspondence with the manually delineated contours and the anatomy in each repeat CBCT scan, respectively. The quantitative evaluation measured the performance of the DIR using a frequently applied similarity measure, the Dice similarity coefficient (DSC) that measures the intersection between pair of segmentations [Citation26]:
The pairs of segmentations in the CBCT sets in this study were the deformed contours from the pCT (VDIR) and the manually delineated contours (VREF) as benchmark. As seen in Equation 1, the DSC was defined by the quotient of the overlap between VDIR and VREF over their mean. The DSC ranges from zero to one where a higher value indicates a larger overlap.
The qualitative evaluation investigated the accuracy of the propagated contours visually, in a slice-by-slice evaluation by a radiation oncologist (LB), not involved in the former delineation process. Each slice was scored based on the number of intolerable shape and/or distance deviations relative to the anatomy. The number of slices with intolerable deviations were categorised in a four-scale classification [Citation25] giving an indication of the quality of the DIR for each VOI and CBCT scan. To investigate any relationship between the qualitative and the quantitative evaluation, the qualitative scoring was associated with the DSC for the corresponding scan.
In IMRT planning optimisation for PC patients at our department, no bladder constraints are used. An additional qualitative evaluation with focus on the deviations within the high-dose region was therefore made. This evaluation considered mainly the performance of the DIR algorithm for the prostate and hence the propagated rectum and bladder contours were only checked to not have major deviations. The deviations for the propagated prostate contours were accepted if the maximal deviations would have been compensated with the routinely applied margin.
Results
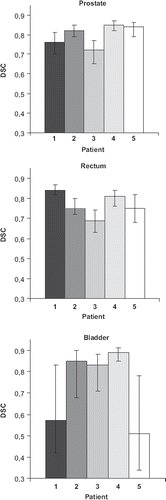
Applying DIR improved the results relative to the rigid registration only, giving higher DSC values for all three organs in more than half of the scans (56%). Considering each organ and scan separately, DIR improved the DSC in 72% of the scans for the prostate, 97% of scans for the rectum and 89% of scans for the bladder. Overall, averaged across all scans and patients, the absolute increase in DSC using DIR was 0.03 for prostate, 0.06 for rectum and 0.09 for bladder (). Applying DIR, also the range in DSC was generally shifted towards higher values, most pronounced for the rectum. The DSC values were highest for prostate, slightly lower for rectum and lowest for bladder ().
Table I. Average DSC (range) for each contour calculated over all 36 registrations for the rigid registration and the DIR. The DSC values for the bladder with the patient exclusion (see text for details) are also shown.
The variation in the DSC across separate scans was smallest for the prostate and largest for the bladder (). Two patients (Patient 1 and 5) had a much smaller bladder in the pCT as compared to all the repeat CBCT scans. Exclusion of these two patients gave an absolute increase of 0.13 in the average DSC and the improvements were even more pronounced with higher values in the range of the DSC for both the DIR and the rigid registration ().
Figure 1. Patient individual DSC averaged over all scans for the prostate, rectum and bladder with corresponding range (error bars).
In the qualitative evaluation there was a large variety in the scores for the prostate while in the majority of the scans the propagated bladder and rectum contours were classified in the less favourable scorings (). The percentage of the propagated contours of good quality, i.e. the scans scored as good together with the scans scored as acceptable was 45% for prostate; 20% for rectum and 33% for bladder (). When associating the scores of the qualitative evaluation with the DSC for the corresponding scan we found an association between the qualitative and the quantitative evaluation for the bladder (), i.e. the DSC decreased with lower qualitative scoring. For the prostate and the rectum we did not find such correspondence.
Figure 2. The number of scans scored according to the qualitative evaluation (good, acceptable, need of adjustment and poor) for each patient and organ, respectively.
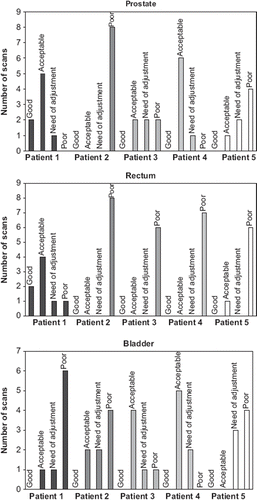
Table II. The qualitative evaluation giving the percentage (number) of scans scored according to the classification in .
Table III. The average DSC (over all scans and patients) for the different scores of the qualitative evaluation.
For the additional qualitative evaluation concentrating on the deviations in the high-dose region, the propagated contours (mainly the propagated prostate contours) were unsatisfying in 39% (14) of the scans. More than half of these scans belonged to one single patient (Patient 2).
Discussion
In this study, we have shown improvements using DIR compared to using rigid registration only for propagation of tumour and OR contours in pelvic RT. In order to validate the DIR algorithm and explore its potential use for daily IGRT as well as for dose accumulation/adaptation [Citation10,Citation17,Citation20], the algorithm was investigated both quantitatively and qualitatively on repeat CBCT scans.
Evaluating the algorithm quantitatively, the DIR was most successful for the prostate while the performance was slightly inferior for the rectum and less satisfying for the bladder (). However, comparing the DSCs with those obtained from the rigid registration the improvement from using DIR was most pronounced for the rectum and bladder. In a recent publication from our institution [Citation25] the DIR algorithm investigated in the present study was evaluated on pCT-CT registrations on another series of PC patients. In comparison with our findings, the DSC values in this previous pCT-CT registration study were somewhat higher for prostate, similar for rectum and much higher for bladder, although the improvements relative the rigid registration were similar. CT-CT registration and contour propagation using a demons algorithm has also been studied by Godley et al., reporting much higher DSCs for the bladder, equivalent for rectum and slightly lower for prostate and with an improvement relative the rigid registration similar to our findings [Citation15].
When considering clinical introduction of a DIR algorithm, a qualitative evaluation allowing a radiation oncologist to visually assess the propagated contours has often been performed [e.g. 6,13]. Our qualitative evaluation confirmed the results from the quantitative evaluation of the algorithm as being most successful for prostate and less successful for rectum and bladder. For the rectum, the inferior qualitative scores and the low DSC values were due to deviations between the pCT and CBCTs in the most cranial parts of this organ. Nevertheless, in RT for PC the cranial part of rectum receives a small fraction of the prescribed dose and is therefore of less clinical importance in terms of dose accumulation and treatment adaptation.
Despite the subjective aspects of a visual assessment it provides a direct way to qualitatively evaluate the DIR when there is no good ground truth [Citation13,Citation21]. However, to explore potential issues with subjectivity with the qualitative evaluations in this study, an additional radiation oncologist was asked to perform this exercise. For all organs (prostate, rectum and bladder), the scoring harmonised with the presented results except for some variance mostly between the ‘acceptable’ and ‘need of adjustment’ scorings for the bladder (data not shown). Hence, different radiation oncologists might perform the qualitative scoring differently but the main and most important patterns (i.e. good and poor) are likely to be captured regardless of observer. Comparing the qualitative evaluation in this study with the results in the study by Thörnqvist et al. [Citation25] using the same scoring system and DIR algorithm indicated improved performance on pCT-CT registrations as the fraction of pCT-CBCT registrations were to a larger extent scored as less favourable.
In an effort to try to associate the DSC with the qualitative scoring, a clear relationship was seen only for the bladder (), which is partially described by the fact that some of the scoring in the qualitative evaluation was missing or belonged to a single patient (). Even with a large DSC value that is supposed to signify the success of the DIR, the agreement with the visual assessment was not always apparent. Similarity measures such as the DSC [Citation26] describes only the agreement between contours and hence might not directly reflect clinical judgement [Citation13]. Quantitative and qualitative validation measures have their advantages and disadvantages yet the common challenge arises from lack of a ground truth [Citation13,Citation17,Citation23,Citation27].
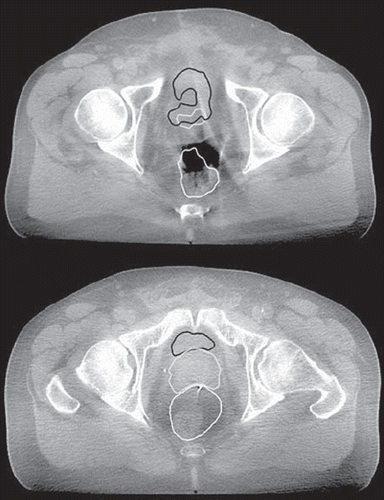
The CBCT scans in this study were extensively influenced by the increased scatter resulting from the CBCT acquisition geometries () which might explain the less successful DIR from pCT to CBCT versus that from pCT to CT. The CBCT scans were acquired in a standard acquisition mode contributing together with different patient positioning and artefacts (from, for example the implanted fiducial markers) to scatter and streak artefacts () [Citation28]. Apart from influencing on the capacity of the DIR algorithm by performing better for scans/patients with less scatter this is likely to complicate the delineation of the organs [Citation2,Citation8,Citation23]. During the manual delineation in particular the most superior and inferior parts of the concerned organs may be difficult to define. In these regions the performance of the DIR was also the least satisfying. The difficulties in defining the organs in the manual delineation in the most superior and inferior parts could explain the lack of VDIR-VREF overlap appearing predominantly in these directions.
Figure 3. The low image quality illustrated in two axial slices of two of the CBCT scans for two different patients (patient 3 - upper figure, patient 2 - lower figure) with the propagated contours from the DIR (prostate grey; rectum white and bladder black) overlaid. In addition the inabilities of the investigated DIR algorithm to handle bowel gas (upper figure) and the artefacts from the fiducial markers (lower figure) are demonstrated.
Previous studies have shown that the inter-observer variations of defining the pelvic organs on CBCT images mainly occur in the superior and inferior parts of the organs [Citation29]. Despite manual delineation as being error-prone it is also a time-consuming procedure [Citation12]. A prerequisite for treatment adaptation would be to have the contours automatically segmented on both the pCT and the CBCT images [Citation5,Citation16,Citation17,Citation23,Citation27,Citation30]. This does not only demand for a reliable DIR algorithm [Citation4,Citation7,Citation27] but also for a unified standard of defining the relevant anatomy [Citation5,Citation31]. In addition, the poor image quality along with the scatter artefacts and the intrafractional motion causing shading and streak artefacts [Citation6,Citation28] may influence on the accuracy of HU [Citation4,Citation28,Citation32] in the CBCT scans. For CBCT-based ART, the HUs need to be calibrated to the electron density for CBCT and the scatter needs to be addressed using, for example accurate algorithms prior to reconstruction to correct for scatter in the raw image projections [Citation19,Citation27,Citation28,Citation32,Citation33]. Another approach of solving the inconsistency in HUs has been presented by Yang et al. [Citation19] where the pCT was deformed to each CBCT scan allowing for dose calculation on each of the HU corrected CBCT scans. The dose distribution obtained from each CBCT scan can be registered back to the pCT allowing for, for example evaluation of the accumulated dose [Citation5,Citation15,Citation17,Citation23,Citation32]. In a future study we will investigate such an approach based on repeat CT scans and subsequently on repeat CBCT scans (though handling the scatter issue) and eventually explore whether this ‘true’ dose distribution will improve our ability to predict normal tissue toxicity relative the planned dose distribution obtained from the pCT.
Two of the patients in this study had a much smaller bladder volume in the pCT (compared to the volume in the repeat CBCT scans), which deteriorated the performance of the DIR. Excluding these patients led to an increase in both the average DSC and a larger improvement compared to using rigid registration only (). Previous studies [Citation2,Citation13,Citation15,Citation25] have also shown that the substantial volume changes occurring in, for example rectum and bladder during the course of the RT is a difficult task for DIR algorithms. Besides, the performance of the investigated DIR algorithm was unsuccessful for the rectum when bowel gas was present on the border of the rectal volume (). A similar phenomenon was also identified for the bladder where in some cases parts of the bowel were classified as bladder. A rigid registration prior to the DIR limits the influence from large deformations [Citation3], nevertheless new objects as for instance air in the rectum or the registration of a full bladder in one image to an empty in another will compromise the ability of the DIR to find a one-to-one correspondence of the images [Citation13,Citation27]. As a result, nearby structures will be greatly deformed if no precaution is given to them [Citation27]. These drawbacks are well known when dealing with particularly intensity-based DIR algorithms [Citation5,Citation13,Citation15,Citation21,Citation23,Citation27]. A focus of future validation studies could therefore be on patients presenting large deformations since these are likely to be the patients where an accurate registration is most needed and challenging. To make DIR more accurate, different approaches have been proposed where the main focus lies on modifying the voxel intensity inside the deformable organs with information from their outer parts or from their contour border [Citation3,Citation15,Citation27]. This has shown to improve the performance of the DIR for the rectum and bladder as well as for the surrounding less deformable organs such as the prostate [Citation13,Citation15,Citation27]. For instance, Godley and colleagues [Citation15] replaced voxels for both rectum and bladder with uniform intensity. This aided the algorithm to find a better correspondence, as indicated by higher DSC values, not only between the bladder and rectum volumes but also for the prostate [Citation15]. Using DIR for ART in this study, the performance for the rectum and bladder needs to be improved [Citation16,Citation29] and for this purpose the above approach might be useful.
In conclusion, this study has documented improvements of DIR over rigid registration for contour propagation from the pCT to a set of CBCT scans for a series of PC patients. However, the study revealed considerable limitations of the current algorithm in handling large deformations. Improved performance of the algorithm – opening the potential for using CBCT scans for dose accumulation and treatment adaptation purposes – is likely to result by improvements of the DIR algorithm as well as improvements of the CBCT image quality.
Acknowledgements
This work has been supported by research grants from CIRRO-The Lundbeck Foundation Center for Interventional Research in Radiation Oncology, the Danish Cancer Society, FSS (The Danish Council for Independent Research) as well as the Danish Council for Strategic Research. Varian Medical Systems (Palo Alto, CA, USA) are acknowledged for providing the DIR application.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Lotz HT, Remeijer P, Van Herk M, Lebesque JV, De Bois JV, Zijp LJ, . A model to predict bladder shapes from changes in bladder and rectal filling. Med Phys 2004;31:1415–23.
- Xie Y, Chao M, Lee P, Xing L. Feature-based rectal contour propagation from planning CT to cone beam CT. Med Phys 2008;35:4450–9.
- Chen T, Kim S, Goyal S, Jabbour S, Zhou J, Rajagopal G, . Object-constrained meshless deformable algorithm for high speed 3D nonrigid registration between CT and CBCT. Med Phys 2010;37:197–210.
- Østergaard Noe K, De Senneville BD, Elstrøm UV, Tanderup K, Sørensen TS. Acceleration and validation of optical flow based deformable registration for image-guided radiotherapy. Acta Oncol 2008;47:1286–93.
- Jaffray DA, Lindsay PE, Brock KK, Deasy JO, Tomé WA. Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. Int J Radiat Oncol Biol Phys 2010;76(Suppl 3):135–9.
- Smitsmans MH, De Bois J, Sonke JJ, Betgen A, Zijp LJ, Jaffray DA, . Automatic prostate localization on cone-beam CT scans for high precision image-guided radiotherapy. Int J Radiat Oncol Biol Phys 2005;63:975–84.
- Grau C, Olsen DR, Overgaard J, Høyer M, Lindegaard JC, Muren LP. Biology-guided adaptive radiation therapy – presence or future? Acta Oncol 2010;49:884–7.
- Van Zijtveld M, Dirkx M, Breuers M, Kuipers R, Heijmen B. Evaluation of the ‘dose of the day’ for IMRT prostate cancer patients derived from portal dose measurements and cone-beam CT. Radiother Oncol 2010;96:172–7.
- Søndergaard J, Høyer M, Petersen JB, Wright P, Grau C, Muren LP. The normal tissue sparing obtained with simultaneous treatment of pelvic lymph nodes and bladder using intensity-modulated radiotherapy. Acta Oncol 2009;48: 238–44.
- Wright P, Muren LP, Høyer M, Malinen E. Evaluation of adaptive radiotherapy of bladder cancer by image-based tumour control probability modelling. Acta Oncol 2010; 49:1045–51.
- Thor M, Væth M, Karlsdottir Á, Muren LP. Rectum motion and morbidity prediction: Improving correlation between late morbidity and DVH parameters through use of rectum planning organs at risk volumes. Acta Oncol 2010;49: 1061–8.
- Yan D, Jaffray DA, Wong JW. A model to accumulate fractionated dose in a deforming organ. Int J Radiat Oncol Biol Phys 1999;44:665–75.
- Gao S, Zhang L, Wang H, de Crevoisier R, Kuban DD, Mohan R, . A deformable image registration method to handle distended rectums in prostate cancer radiotherapy. Med Phys 2006;33:3304–12.
- Webb S. The contribution, history, impact and future of physics in medicine. Acta Oncol 2009;48:169–77.
- Godley A, Ahunbay E, Peng C, Li XA. Automated registration of large deformations for adaptive radiation therapy of prostate cancer. Med Phys 2009;36:1433–41.
- Schaly B, Kempe JA, Bauman GS, Battista JJ, Van Dyk J. Tracking the dose distribution in radiation therapy by accounting for variable anatomy. Phys Med Biol 2004;49: 791–805.
- Wang H, Dong L, O'Daniel J, Mohan R, Garden AS, Ang KK, . Validation of an accelerated ‘demons’ algorithm for deformable image registration in radiation therapy. Phys Med Biol 2005;50:2887–905.
- Gu X, Pan H, Liang Y, Castillo R, Yang D, Choi D, . Implementation and evaluation of various demons deformable image registration algorithms on a GPU. Phys Med Biol 2010;55:207–19.
- Yang Y, Schreibmann E, Li T, Wang C, Xing L. Evaluation of on-board kV cone beam CT (CBCT)-based dose calculation. Phys Med Biol 2007;52:685–705.
- Yan D, Vicini F, Wong J, Martinez A. Adaptive radiation therapy. Phys Med Biol 1997;42:123–32.
- Lu W, Olivera GH, Chen Q, Ruchala KJ, Haimerl J, Meeks SL, . Deformable registration of the planning image (kVCT) and the daily images (MVCT) for adaptive radiation therapy. Phys Med Biol 2006;51:4357–74.
- Fellin G, Fiorino C, Rancati T, Vavassori V, Baccolini M, Bianchi C, . Clinical and dosimetric predictors of late rectal toxicity after conformal radiation for localized prostate cancer: Results of a large multicenter observational study. Radiother Oncol 2009;93:197–202.
- Chao M, Xie Y, Xing L, Auto-propagation of contours for adaptive prostate radiation therapy. Phys Med Biol 2008;53:4533–42.
- Thirion JP. Image matching as a diffusion process: An analogy with Maxwell's demons. Med Image Anal 1998;2:243–60.
- Thörnqvist S, Petersen JBB, Høyer M, Bentzen LN, Muren LP. Propagation of target and organ at risk contours in radiotherapy of prostate cancer using deformable image registration. Acta Oncol 2010;49:1023–32.
- Dice LR. Measures of the amount of ecologic association between species. Ecology 1945;26:297–302.
- Yang D, Chaudhari SR, Goddu SM, Pratt D, Khullar D, Deasy JO, . Deformable registration of abdominal kilovoltage treatment planning CT and tomotherapy daily megavoltage CT for treatment adaptation. Med Phys 2009;36:329–38.
- Zhu L, Xie Y, Wang J, Xing L. Scatter correction for cone-beam CT in radiation therapy. Med Phys 2009;36:2258–68.
- White EA, Brock KK, Jaffray DA, Catton CN. Inter-observer variability of prostate delineation on cone beam computerised tomography images. Clin Oncol 2009;21:32–8.
- Huyskens DP, Maingon P, Vanuytsel L, Remouchamps V, Roques T, Dubray B, . A qualitative and a quantitative analysis of an auto-segmentation module for prostate cancer. Radiother Oncol 2009;90:337–45.
- Allozi R, Li XA, White J, Apte A, Tai A, Michalski JM, . Tools for consensus analysis of experts’ contours for radiotherapy structure definitions. Radiother Oncol 2010;97:572–8.
- Guan H, Dong H. Dose calculation accuracy using cone-beam CT (CBCT) for pelvic adaptive radiotherapy. Phys Med Biol 2009;54:6239–50.
- Elstrøm UV, Wysocka BA, Muren LP, Petersen JB, Grau C. Daily kV cone-beam CT and deformable image registration as a method for studying dosimetric consequences of anatomic changes in adaptive IMRT of head and neck cancer. Acta Oncol 2010;49:1101–8.