Abstract
Introduction. Vascular endothelial growth factor (VEGF) may play a role in erythropoiesis. We performed a meta-analysis of randomized controlled trials (RCT) to determine the effect of the anti-VEGF antibody bevacizumab on anemia in cancer patients treated with chemotherapy. Methods. Databases from PUBMED, the Web of Science, Embase, the Cochrane Library, and abstracts presented at the American Society of Clinical Oncology (ASCO) conferences until May 2010 were searched to identify relevant studies. Eligible studies included prospective RCTs in which the combination of bevacizumab and chemotherapy was compared with chemotherapy alone. Summary incidence rate, relative risk (RR), and 95% confidence interval (CI) were calculated. Results. A total of 6439 patients with a variety of solid tumors were included for analysis from 11 RCTs. Among those patients receiving bevacizumab and chemotherapy, the incidences of all-grade and high-grade (grade 3 and above) anemia were 17.8% (95% CI: 11.1–27.1%) and 2.8% (95% CI: 1.6–5.0%) respectively. In comparison with chemotherapy alone, bevacizumab significantly reduced all-grade (RR, 0.79; 95% CI: 0.66–1.0, p = 0.007) and high-grade anemia (RR, 0.72; 95% CI: 0.57–0.90, p = 0.005). The effect did not vary significantly among bevacizumab doses (p = 0.88), tumor types (p = 0.75) or chemotherapy regimens (p = 0.98). Discussion. Bevacizumab may significantly reduce the risk of anemia with chemotherapy in cancer patients.
Vascular endothelial growth factor (VEGF) plays an important role in tumor growth and metastasis by promoting angiogenesis, and the blockade of its signaling pathway has become a major approach to current cancer treatment [Citation1]. Bevacizumab (Avastin, Genentech Inc, South San Francisco, CA), a humanized monoclonal antibody against VEGF-A, has been successfully developed and used extensively for the treatment of several advanced cancers, including colorectal cancer, non-small cell lung cancer (NSCLC), breast cancer, renal cell cancer, and glioblastoma multiforme [Citation2].
In addition to its role in promoting tumor progression [Citation3], abnormal expression of VEGF can systematically impair multiple organs, resulting in defective hematopoiesis and endocrine functions, and early death in tumor-bearing mice [Citation4]; the use of anti- VEGF and VEGFR antibodies significantly improved hematopoiesis and cancer-associated systeic syndrome, and overall survival [Citation5]. In addition, VEGF may be a key negative regulator of hepatic erythropoietin synthesis and erythropoiesis; increased production of red blood cells occurred in both mouse and primate models after the high-grade inhibition of VEGF by adenoviral expression of soluble VEGF receptor (VEGFR), recombinant VEGF trap protein, and an VEGFR2-selective antibody; further studies showed that the inhibition of VEGF induced a dramatic hepatic synthesis of erythropoietin > 40-fold [Citation6]. Consistent with these findings, several retrospective studies in cancer patients have observed that the use of VEGF inhibitors including bevacizumab, sunitinib, sorafenib, and axitinib was associated with an increase in hemoglobin level or secondary erythrocytosis [Citation7–11]. However, these clinical studies are limited due to a lack of controls and retrospective nature.
High-grade anemia, including grade 3 (Hb: 6.5 to 8 g/dl), grade 4 (Hb < 6.5 g/dl) and grade 5 (death), causes significant symptoms, requires transfusion and treatment interruption, and even mortality. In order to determine the effect of VEGF inhibition on erythropoiesis, we hypothesized that the anti-VEGF antibody bevacizumab may significantly reduce the risk of anemia commonly associated with chemotherapy in cancer patients. Because individual trial may be underpowered to assess the impact of bevacizumab on the risk of anemia, we conducted a systematic review and a meta-analysis of randomized trials in which bevacizumab in combination with chemotherapy was compared to chemotherapy alone.
Methods
Data source
The citations from Pubmed between January 1966 and May 2010 were searched using the keywords bevacizumab or avastin, and cancer. Our search was limited to randomized controlled trials (RCTs) without language restriction. We also searched abstracts and virtual meeting presentations from the American Society of Clinical Oncology conferences (http://www.asco.org/ASCO) held between January 2000 and May 2010 for additional studies. In addition, we included text terms such as vascular endothelial growth factor, angiogenesis, black box warnings, side effects, anemia, erythropoiesis, and erythropoietin for relevant information. To ensure completeness of search strategy, we independently searched the citations using databases from the Web of Science, Embase, and the Cochrane library for all relevant RCTs. We reviewed FDA submission documents, the updated manufacturer's package insert, pertinent drug letters, editorial comments, and relevant review articles. The reference lists of the trials as well as articles were reviewed for additional publications. When there was duplication of publications, we reviewed each article, and included only the most recent or the complete version of the trial for analysis. In situations where there was a discrepancy in the data, we considered safety report from the most recent package insert to be most accurate, and used it instead of original publication for our meta-analysis.
Study Selection
The objective of this study was to determine the impact of bevacizumab on the risk of anemia in patients with cancer. Thus, we only included RCTs in which bevacizumab in combination with chemotherapy was compared with chemotherapy alone. Phase 1 trials and single-arm phase 2 trials were excluded due to the lack of controls. Specifically, clinical trials that met the following criteria at the level of individual studies were included in the meta-analysis: (1) prospective phase 2 and 3 RCTs of patients with solid tumors; (2) randomization to the combination of bevacizumab and chemotherapy or chemotherapy alone; and (3) available data including event or incidence of anemia. Quality was assessed using criteria including adequate blinding of randomization, completeness of follow-up, and objectivity of outcome measurements [Citation12].
Data extraction and clinical end points
We extracted details on study characteristics, number of patients, study sponsors, patient characteristics, treatment information, and follow-up from the selected trials. The data of anemia was gathered from the safety profile of each trial. All authors extracted the data independently, and resolved discrepancies by consensus. The occurrence of anemia in these studies was assessed and recorded according to the National Cancer Institute's common terminology criteria for adverse events (version 2 or 3), which has been widely used in cancer clinical trials [Citation13,Citation14]. The major difference between these two versions includes the addition of grade 5 (death) in version 3 ().
Table I. National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Versions 2 and 3 for anemia.
Statistical analysis
Version 2 of the Comprehensive Meta-analysis program (Biostat, Englewood, NJ) was used for statistical analysis. The incidence of anemia was calculated by using the number of patients with anemia in the bevacizumab group and the total number of patients receiving bevacizumab. The proportion of patients with anemia and 95%CI was extracted from each trial. The relative risk was calculated by comparing the patients assigned to bevacizumab group with control group. In order to explore the dose-effect relationship, we also classified bevacizumab therapy into low dose (5 or 7.5 mg/kg per dose per schedule, which is equivalent to 2.5 mg/kg per week) and high dose (10 or 15 mg/kg per dose per schedule, which is equivalent to 5 mg/kg per week). We have previously shown the dose-dependent risk of hypertension and dose-independent risk of venous thromboembolism with bevacizumab [Citation15–17].
We used both fixed-effects (weighted with inverse variance) and random-effects models for our meta-analysis [Citation18]. For each meta-analysis, the Cochran's Q statistics and I2 statistics were first calculated to assess the heterogeneity among the proportions of the included trials [Citation19]. For the p-value < 0.1, the assumption of homogeneity was deemed invalid, and the random-effects model was reported after exploring the causes of heterogeneity. Otherwise, the fixed-effects model was reported. We used the Begg's and Egger's tests to determine the presence of publication bias regarding the primary end point (RR of anemia) [Citation20,Citation21]. A two-tailed p-value < 0.05 was considered statistically significant.
Results
Search results
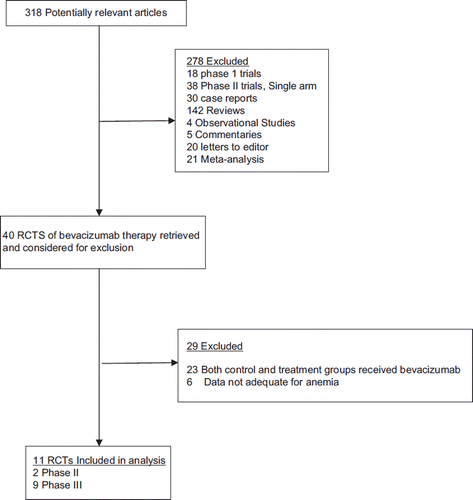
Our search yielded a total of 318 potentially relevant clinical studies on bevacizumab (see for the selection process of these studies). We excluded review articles, observational studies, case reports, meta-analysis, single-arm phase 2 studies, and RCTs with both arms containing bevacizumab. A total of 11 RCTs (2 phase II and 9 phase III trials) were included in this analysis, in which the combination of bevacizumab with chemotherapy was compared to chemotherapy alone ().
Table II. Characteristics of randomized controlled trials included in the meta-analysis.
Study quality
Patients were enrolled according to pre-specified eligibility criteria for each trial. Randomized treatment allocation sequences were generated in all trials. Six trials had the double-blind placebo controlled design [Citation22–27], one trial had placebo as control [Citation28], and the rest of the trials had open-labeled controls [Citation29–32]. Anemia was assessed and recorded according to the National Cancer Institute's common toxicity criteria version 2 or 3. Version 2 was used in four trials [Citation28–31], and version 3 was also used in four trials [Citation22,Citation26,Citation27,Citation32], while the rest did not specify. All trials had sufficient follow-up. The quality of all trials was considered adequate.
Publication bias
No evidence of publication bias was detected for the primary endpoint of this study (RR of high-grade anemia) by either Begg's or Egger's test (Begg's test, p = 0.41; Egger's test, p = 0.43).
Patients
A total of 6439 patients with various solid tumors from 11 RCTs were included for analysis. The Eastern Cooperative Oncology Group (ECOG) status for most of the patients was between 0 and 1. The patients were required to have adequate functions of major organs and hematological profiles. However, the baseline hemoglobin levels were not specified in any of these trials. The underlying malignancy profile of the patient population included lung cancer (three trials), breast cancer (three trials), renal cell carcinoma (two trials), pancreatic cancer (two trials), and malignant mesothelioma (1 trial).
Patients with significant cardiovascular disease, peripheral vascular disease, uncontrolled hypertension, serious non-healing wounds, pregnant and lactating women, major surgery within the past 28 days, pre-existing bleeding diathesis, and brain metastasis were excluded. Moreover, patients taking regular aspirin (>325 mg/day), NSAIDs, and oral or parenteral anticoagulants (with the exception of prophylactic anticoagulants to maintain vascular device access), were also excluded. The use of growth factors including erythropoietin during chemotherapy was not specified.
Incidence of all-grade and high-grade anemia
All-grade anemia is defined as a hemoglobin level less than the lower limit of normal (man < 14.0 g/dl, woman < 12.0 g/dl). Meta-analysis showed that the incidence of all-grade anemia among 1073 patients was 17.8% (95% CI: 11.1–27.1%) according to a random-effects model (Q = 31.89, p < 0.001, I2 = 90.59). No grade 5 anemia (death) was reported. Meta-analysis showed that the incidence of high-grade anemia was 2.9% (95% CI: 1.6–5.0%) among 3580 patients from all 11 RCTs according to the random-effects model (Q = 74.45, p < 0.001, I2 = 86.57).
Relative risk of anemia with bevacizumab
In order to determine the particular effect of bevacizumab on anemia, we calculated the relative risk of bevacizumab in combination with chemotherapy in comparison with chemotherapy alone for each RCT and performed a meta-analysis for its overall effect. The summary incidence of all-grade anemia in 980 patients treated with chemotherapy alone was 21.8% (95% CI: 14.1–32.3%) based on four RCTs [Citation22,Citation26,Citation28,Citation33], according to the random-effects model (Q = 31.35, p < 0.001, I2 = 90.43). When compared to chemotherapy alone, patients receiving the combination of bevacizumab and chemotherapy has a reduced risk of all-grade anemia with a summary RR of 0.79 (95% CI: 0.66–0.94, p = 0.007; ), according to the fixed-effects model (Q = 0.84, p = 0.83, I2 < 0.001). This result suggested that the addition of bevacizumab to chemotherapy significantly reduced the risk of anemia by 21%.
Figure 2. Relative risk (RR) of anemia with bevacizumab compared with control. Both fixed- and random-effects models were used to calculate relative risks. Overall relative risks of all-grade (A) and high-grade (B) anemia were reported using the random and fixed -effects models. The incidence of anemia was compared between patients treated with bevacizumab in combination with chemotherapy (bevacizumab) and chemotherapy alone (control) in each study. RR for each study is displayed numerically on the left and graphically on the right. Total events and sample sizes are also displayed for each study. For study name, the first author's name was used to represent each trial. If the same first author was involved in two trials, then the publication year was also included to identify the trial. For each trial the position of the square denoted the value of RR, horizontal lines represent 95% CI, and diamond plot represents overall results of the included trials.
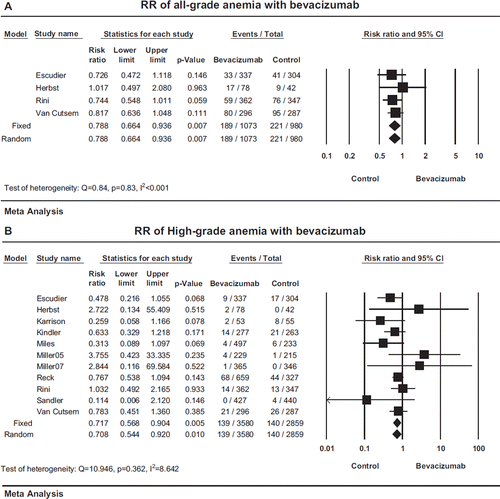
The summary incidence of high-grade anemia in 2859 patients treated with chemotherapy alone was 4.4% (95% CI: 2.6–7.4%) based on all 11 RCTs, according to the random-effects model (Q = 73.52, p < 0.001, I2 = 86.40). In comparison with chemotherapy alone, bevacizumab significantly decreased the risk of high-grade anemia associated with chemotherapy with an RR of 0.72 (95% CI 0.57–0.90, p = 0.005; ), according to the fixed-effects model (test of heterogeneity: Q = 10.95, p = 0.36, I2 = 8.64), suggesting a 28% risk reduction.
Effect of bevacizumab dose on high-grade anemia
To further understand the role of bevacizumab in decreasing chemotherapy-associated anemia, we explored the relationship between the dose of bevacizumab and RR of high-grade anemia. The RR for bevacizumab at 5 mg/kg/week was 0.71 (95% CI: 0.54–0.94, p = 0.015) as calculated from 10 RCTs according to the fixed-effects model, suggesting that bevacizumab significantly decreased the risk of high-grade anemia at the high dose. The RR for bevacizumab at 2.5 mg/kg/week was 0.74 (95% CI: 0.53–1.03, p = 0.077) as calculated from three RCTs according to the fixed- effects model, suggesting a trend favoring that bevacizumab reduced the risk of high-grade anemia. No significant difference was observed between the two dose levels in the RR of high-grade anemia (p = 0.88).
Tumor type and the effect of bevacizumab
Tumor type may have a role in the development of high-grade anemia due to underlying biology including VEGF expression or polymorphism, and related treatment. Thus, we calculated the risk of high-grade anemia separately according to tumor histology (). The incidence of high-grade anemia with bevacizumab ranged from 0.9% in breast cancer to 6.2% in pancreatic cancer, with a significant variation among various tumors (p = 0.001), suggesting the absolute risk of high-grade anemia in these patients was influenced by underlying cancer or its associated treatment.
Table III. Incidence and relative risk (RR) of high-grade anemia with bevacizumab among patients with various tumor types.
RRs of high-grade anemia with bevacizumab ranged from 0.26 (95% CI: 0.06–1.17) for mesothelioma to 1.1 (95% CI: 0.18–7.0) for breast cancer (). However, no significant difference was found among these tumors (p = 0.78), suggesting that the bevacizumab effect may be independent of underlying cancer types.
Chemotherapy regimen and the effect of bevacizumab
We also performed an analysis of the risk of high-grade anemia according to chemotherapy regimen that patients received. The addition of bevacizumab to chemotherapy reduced the risk of high-grade anemia similarly in patients undergoing single agent versus doublet combination (p = 0.98), platinum versus non-platinum (p = 0.43). Thus, the effect of bevacizumab was not affected by chemotherapy regimen significantly.
Risk of high-grade anemia and clinical outcome
A small case series revealed that increase in hemoglobin levels was associated with longer progression-free survival (PFS), and may be a surrogate marker for the efficacy of VEGF inhibition [Citation9]. Thus, we looked into the relationship between high-grade anemia and clinical outcome such as PFS and OS (overall survival) at study level. No significant correlation was seen between RR of high-grade anemia and hazard ratios of PFS (r2 = 0.036, p = 0.69) in these studies. Similarly, we did not find any correlation between RR of high-grade anemia and hazard ratio of OS (r2 = 0.021, p = 0.64). Therefore, there appears no significant correlation between the effect of bevacizumab on high-grade anemia and clinical outcome at study level.
Discussion
To our knowledge, this analysis is the first study to reveal that an VEGF inhibitor significantly reduced the risk of anemia in cancer patients treated with chemotherapeutic agents. We have demonstrated that the anti-VEGF antibody bevacizumab significantly reduced the risk of high-grade anemia (RR, 0.72; 95% CI: 0.57–0.90, p = 0.005) as well as all-grade anemia (RR, 0.79; 95% CI: 0.66–0.94, p = 0.007). The risk reduction of serious anemia by bevacizumab may be relatively comparable to that of erythropoiesis-stimulating agent (ESAs), which decreased transfusion requirement with an RR of 0.64 (95% CI: 0.60–0.68) according to a meta-analysis of 57 studies including 9353 patients [Citation34]. Although the use of erythropoiesis-stimulating agent (ESAs) has decreased the risk of anemia and transfusion needs, recent concerns have arisen about increased mortality, venous thromboembolism, and tumor progression associated with their use [Citation35–37], and FDA has issued a black box warning regarding the increased risk in cancer patients [Citation38]. This underscores the importance of devising novel combination regimens and alternative therapeutic strategies to overcome this problem. The use of bevacizumab may reduce the need for ESAs or transfusion in cancer patients treated with chemotherapy; in addition, high-grade anemia may cause the treatment interruption and discontinuation of chemotherapy, thus compromising the efficacy of cancer therapy. Therefore, bevacizumab may improve cancer treatment not only by its anti-angiogenesis effect but also by decreasing the occurrence of high-grade anemia and increasing the administration of chemotherapy.
While the exact mechanism underlying the effect of bevacizumab on anemia remains unclear, several possibilities related to VEGF inhibition may exist. The reduced risk of anemia may be secondary to the increased production of endogenous erythropoietin associated with VEGF inhibition. Indeed, high-grade VEGF inhibition by a variety of inhibitors has been shown to promote hepatic erythropoietin (EPO) synthesis and erythrocytosis in preclinical models [Citation6,Citation39]; also, bevacizumab may cause tissue hypoxia due to its anti-angiogenesis and vasoconstriction effect, leading to subsequent up-regulation of erythropoietin. Alternatively, the erythropoietic effect of bevacizumab may be independent of erythropoietin and related to its anti-VEGF or anti-tumor effect. It has been shown that tumor-derived VEGF was sufficient to cause profound bone marrow suppression in mice [Citation4], and the VEGF neutralization by bevacizumab normalized hematopoiesis in the absence of controlling tumor growth [Citation5]; in addition, bevacizumab may improve hematopoiesis by its enhanced anti-tumor effect of bone-marrow infiltrative disease in combination with chemotherapy.
The effect of VEGF inhibitors on erythropiesis may depend on its target specificity. Bevacizumab as a specific antibody against VEGF-A was found to increase hemoglobin at a median of 1.4 g/dl (median percent rise: 12.3%) in the majority (90%) of patients with RCC [Citation9]. For multiple tyrosine kinase inhibitors such as sorafenib and sunitinib, which blocked the activity of VEGFR 1-3, c-Kit, and other receptor tyrosine kinase, the hemoglobin level may be determined by the balance between the VEGF blockage, which induces erythrocytosis, and c-kit inhibition, which may cause anemia [Citation8]. These agents have been shown to be associated with anemia in phase III trials [Citation40–42]. On the other hand, they were found to induce secondary erythrocytosis in some cancer patients [Citation8,Citation11]. In addition, axitinib, a potent VEGFR inhibitor, was found to induce secondary polycythemia, which was confirmed by isotopic measurement of red cell mass and the repeat need for phlebotomy [Citation7]. Further more, cyclic variations in hemoglobin level were found to parallel the administration of sunitinib in 82 patients with metastatic RCC, suggesting a temporary loss of intravascular fluid by inhibition of VEGFR-2 could partly contribute to an increase in hematocrit [Citation10].
The absolute risk of high-grade anemia associated with bevacizumab ranged from 0.9% (95% CI: 0.4–2.2%) in breast cancer to 6.2% (95% CI: 4.4–8.5%) in pancreatic cancer, and varied significantly among tumor types (p = 0.001). The variation may result from the biology of underlying tumor and associated treatment. However, we found that the risk reduction of bevacizumab on high-grade anemia did not vary significantly among tumor types (p = 0.78). It is possible that the effect of bevacizumab may be comparable among advanced cancers irrespective of tumor types due to similarly increased VEGF levels and comparable high-grade VEGF inhibition. Alternatively, our result may be limited by relatively small sample size for each tumor type. The risk reduction of anemia also did not vary significantly among different chemotherapies, suggesting that the effect of bevacizumab may be independent of chemotherapy regimen used in these studies.
The risk reduction of high-grade anemia did not differ significantly between bevacizumab at 2.5 and 5 mg/kg/week (p = 0.88). One possible explanation is that the high-grade VEGF inhibition required to promote erythopoiesis may be achieved at 2.5 mg/kg/week, and further increase in doses may not lead to increased erythropoiesis. Alternatively, this analysis may not be powered to show a difference in anemia reduction with these doses.
In addition to its impact on morbidity and the quality of life, anemia is an independent prognostic factor for survival in cancer patients [Citation43]. An association between the degree of hemoglobin increase and longer PFS in RCC patients undergoing anti-VEGF treatment was observed by a small retrospective study [Citation9]. The effect of bevacizumab on anemia as a biomarker of cancer treatment efficacy will need further evaluation. At study level, we did not find significant correlation between the RR of high-grade anemia and HR of PFS or OS. However, this does not exclude the possibility that there might be a correlation at individual patient level. Further studies will be needed to understand the risk factors influencing the bevacizumab effect on anemia. In addition, serial measurements of reticulocyte counts during therapy might start to shed some light on mechanisms by which bevacizumab protects against (or prevents) chemotherapy-related anemia.
Our study has several limitations. Firstly, the reporting of all-grade anemia was limited to 4 of 11 studies, and for high-grade anemia, it was reported in combined grades (grades 3–4). This has not allowed us for more extensive sub-group analysis. In addition, no baseline hemoglobin level was mentioned in any studies. Secondly, there was significant heterogeneity in the incidences of anemia among the included studies. This may reflect differences in sample sizes, medication doses, tumor type, concomitant chemotherapy and other factors among these studies. We tried to minimize this bias by using a random–effects model to calculate the overall incidence of all-grade and high-grade anemia. Despite these differences, the RRs reported by all these studies showed remarkable non-heterogeneity (p = 0.36). Thirdly, these studies were mostly conducted at academic centers and major research institutions, and the patients included had adequate major organ function, which might not reflect patient populations in the community and in the setting of organ dysfunction. Fourthly, this is a meta-analysis at the study level, and confounding factors at the patient level could not be assessed properly and incorporated into the analysis. Finally, the study might have under-estimated the effect of bevacizumab on anemia; a potential bias in observation time and chemotherapy exposure existed because bevacizumab groups might have longer follow-up time and more chemotherapy administration than controls owing to the prolonged PFS that is often associated with the use of bevacizumab. In fact, adjusted RRs according to PFS showed more profound impact of bevacizumab on the risk of all-grade anemia (RR: 0.56, 95% CI: 0.46–0.65) and high-grade anemia (RR: 0.58, 95% CI: 0.46–78).
In conclusion, our analysis of randomized controlled trials has shown that bevacizumab significantly reduced the risk of chemotherapy-associated anemia in cancer patients. The result supports the notion that VEGF is a negative regulator of erythropoiesis, and its inhibitors may have a role in the treatment of anemia. The reduction of high-grade anemia may decrease the need of transfusion or ESA use in cancer patients while undergoing chemotherapy. Further studies are strongly recommended to prospectively investigate the effect of VEGF inhibitors on erythrocytosis and hemoglobin change as a biomarker for anti-VEGF therapy.
Acknowledgements
The study was partially funded by the Research Foundation of SUNY at Stony Brook. It has not played any role in design, collection, analysis, interpretation, and writing of the study. SW conceived the study, and was responsible for data analysis. All authors contributed to data collection, literature search, writing, tables, and figures. S.W had full access to all of the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Declaration of interest: S.W. received honoraria from Onyx Pharmaceuticals, Genentech Novartis, and is a speaker for Onyx, Pfizer, and Novartis. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 2004;3:391–400.
- Genentech. Bevacizumab prescribing information. 2009.
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76.
- Xue Y, Chen F, Zhang D, Lim S, Cao Y. Tumor-derived VEGF modulates hematopoiesis. J Angiogenes Res 2009;1:9.
- Xue Y, Religa P, Cao R, Hansen AJ, Lucchini F, Jones B, . Anti-VEGF agents confer survival advantages to tumor-bearing mice by improving cancer-associated systemic syndrome. Proc Natl Acad Sci USA 2008;105:18513–8.
- Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, . VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med 2006;12:793–800.
- Alexandre I, Billemont B, Meric JB, Richard S, Rixe O. Axitinib induces paradoxical erythropoietin synthesis in metastatic renal cell carcinoma. J Clin Oncol 2009;27:472–3; author reply 3–4.
- Alexandrescu DT, McClure R, Farzanmehr H, Dasanu CA. Secondary erythrocytosis produced by the tyrosine kinase inhibitors sunitinib and sorafenib. J Clin Oncol 2008;26:4047–8.
- Harshman LC, Kuo CJ, Wong BY, Vogelzang NJ, Srinivas S. Increased hemoglobin associated with VEGF inhibitors in advanced renal cell carcinoma. Cancer Invest 2009;27:851–6.
- van der Veldt AA, Boven E, Vroling L, Broxterman HJ, van den Eertwegh AJ, Haanen JG. Sunitinib-induced hemoglobin changes are related to the dosing schedule. J Clin Oncol 2009;27:1339–40; author reply 40–2.
- Richard S, Croisille L, Yvart J, Casadeval N, Eschwege P, Aghakhani N, . Paradoxical secondary polycythemia in von Hippel-Lindau patients treated with anti-vascular endothelial growth factor receptor therapy. Blood 2002;99: 3851–3.
- Meade MO, Richardson WS. Selecting and appraising studies for a systematic review. Ann Intern Med 1997;127:531–7.
- NCI [Internet]. National cancer institute's common toxicity criteria (Version 2). [cited 2010 Apr 2]. Available from: http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcv20_4-30-992.pdf.
- NCI [Internet]. National cancer institute's common toxicity criteria (Version 3.0). [cited 2010 Apr 2]. Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
- Ranpura V, Pulipati B, Chu D, Zhu X, Wu S. Increased risk of high-grade hypertension with bevacizumab in cancer patients: A meta-analysis. Am J Hypertens Epub 2010 Feb 25.
- Nalluri SR, Chu D, Keresztes R, Zhu X, Wu S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA 2008;300:2277–85.
- Zhu X, Wu S, Dahut WL, Parikh CR. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis 2007;49:186–93.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58.
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50: 1088–101.
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34.
- Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, . Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet 2007;370:2103–11.
- Karrison T, Kindler HL, Gandara DR, Lu C, Guterz TL, Nichols K, . Final analysis of a multi-center, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin (GC) plus bevacizumab (B) or placebo (P) in patients (pts) with malignant mesothelioma (MM). J Clin Oncol (Meeting Abstracts) 2007;25(18 suppl):7526.
- Kindler HL, Niedzwiecki D, Hollis D, Oraefo E, Schrag D, Hurwitz H, . A double-blind, placebo-controlled, randomized phase III trial of gemcitabine (G) plus bevacizumab (B) versus gemcitabine plus placebo (P) in patients (pts) with advanced pancreatic cancer (PC): A preliminary analysis of Cancer and Leukemia Group B (CALGB). J Clin Oncol (Meeting Abstracts) 2007;25(18 suppl):4508.
- Miles D, Chan A, Romieu G, Dirix LY, Cortes J, Pivot X, . Randomized, double-blind, placebo-controlled, phase III study of bevacizumab with docetaxel or docetaxel with placebo as first-line therapy for patients with locally recurrent or metastatic breast cancer (mBC): AVADO. J Clin Oncol (Meeting Abstracts) 2008;26(15 suppl):LBA1011.
- Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, . Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol 2009;27: 2231–7.
- Reck M, von Pawel J, Zatloukal P, Ramlau R, Gorbounova V, Hirsh V, . Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small cell lung cancer: AVAil. J Clin Oncol 2009;27:1227–34.
- Herbst RS, O'Neill VJ, Fehrenbacher L, Belani CP, Bonomi PD, Hart L, . Phase II study of efficacy and safety of bevacizumab in combination with chemotherapy or erlotinib compared with chemotherapy alone for treatment of recurrent or refractory non small-cell lung cancer. J Clin Oncol 2007;25:4743–50.
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, . Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. New Engl J Med 2006;355:2542–50.
- Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, . Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 2005;23:792–9.
- Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, . Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med 2007;357: 2666–76.
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou S-S, . Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008;26:5422–8.
- Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, . Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: Final results of CALGB 90206. J Clin Oncol 2010;28:2137–43.
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, . Recombinant human erythropoietins and cancer patients: Updated meta-analysis of 57 studies including 9353 patients. J Natl Cancer Inst 2006;98:708–14.
- Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, . Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemia. JAMA 2008;299:914–24.
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, . Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: A meta-analysis of randomised trials. Lancet 2009;373:1532–42.
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, . Erythropoietin or Darbepoetin for patients with cancer – meta-analysis based on individual patient data. Cochrane Database Syst Rev 2009(3):CD007303.
- Amgen [Internet]. Epogen (Epoetin alpha) for injection. [cited 2010 Jul 21]. Available from: http://pi.amgen.com/united_states/epogen/epogen_pi_hcp_english.pdf
- Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis 2004;7:335–45.
- Munugalavadla V, Kapur R. Role of c-Kit and erythropoietin receptor in erythropoiesis. Crit Rev Oncol Hematol 2005;54: 63–75.
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, . Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125–34.
- Hartmann JT, Haap M, Kopp HG, Lipp HP. Tyrosine kinase inhibitors – A review on pharmacology, metabolism and side effects. Curr Drug Metab Epub 2009 Jun 1.
- Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer 2001;91:2214–21.