Abstract
Background. In vitro RBE values for various high LET radiation types have been determined for many different cell types. Occasionally it is criticized that RBE for a given endpoint cannot be single-value dependent on LET alone, but also on particle species, due to the different dose deposition profiles on microscopic scale. Hence LET is not sufficient as a predictor of RBE, and this is one of the motivations for development of radiobiological models which explicitly depend on the detailed particle energy spectrum of the applied radiation field. The aim of the present study is to summarize the available data in the literature regarding the dependency of RBE on LET for different particles. Method. As RBE is highly dependent on cell type and endpoint, we discriminated the RBE-LET relationship for the three investigated cell lines and at the same endpoint (10% survival in colony formation). Data points were collected from 20, four and four publications for V79, CHO and T1, respectively, in total covering 228 RBE values from a broad range of particle species. Results and discussion. All RBE-LET data points demonstrate surprising agreement within the general error band formed by the numerous data points, and display the expected RBE peak at around 100–200 keV/μm. For all three cell lines, the influence of varying the particle type on the RBE was far from obvious, compared to the general experimental noise. Therefore, a dependence of particle type cannot be concluded, and LET alone in fact does seem to be an adequate parameter for describing RBE at 10% survival.
High linear energy transfer (LET) radiation is characterized by a higher biological effectiveness compared to photons of low LET. Because high LET radiation is densely ionizing, the correlated damages of the DNA structure within one cell occur more often so that it becomes more difficult for the cell to repair the damage, leading to a markedly increased efficiency of cell killing [Citation1]. The concept of relative biological effectiveness (RBE) has been introduced to account for this increased efficiency. RBE is defined as the ratio of a dose of photons to a dose of any other particle to produce the same biological effect. High LET beams may have RBEs ranging from 1.5 to 3 [Citation2].
RBE values for various high LET radiation types have been determined for many different cell types, both in vitro and in vivo. It has been demonstrated in in vitro studies that RBE is highly dependent on both cell type and the studied endpoint [Citation3], but also on particle species, due to the different dose deposition profiles on microscopic scale [Citation4,Citation5].
Hence LET is not sufficient as a predictor of RBE. This is one of the motivations for the development of radiobiological models which explicitly depend on the detailed particle energy spectrum of the applied radiation field. Several models exist which aim to predict the biological response of cells irradiated with high-LET radiation. The most prominent models in radiotherapy context are based on the amorphous track formalism established by Butts and Katz [Citation6]. These models explicitly point out that the response of a biological system cannot be characterized with LET as a single valued parameter. The aim of the present study is to review the available data in the literature regarding the dependency of RBE on LET for different particles for three different cell lines at the same endpoint (survival fraction at 10%).
Method
By means of a MEDLINE search (September 2010), 52 papers were found that investigated the relationship between LET and RBE. The MEDLINE search was based on the keywords: LET and RBE. Additional publications were located using citations within the identified papers. All together 838 RBE data point were found reported. From these, data points from studies with the endpoint clonogenic survival (survival fraction at 10%), for the three cell lines V79 (Chinese hamster lung fibroblasts), CHO (Chinese hamster ovarian cells) and T1 (human kidney cells) were included. With these criteria, 26 papers were selected, studying various particle species in the LET range between 7 keV/μm and 2000 keV/μm.
The data is both from publications using monoenergetic beams, quasi-monoenergetic degraded beams, and Spread Out Bragg Peaks. There are two methods to calculate average LET: dose-averaged LET and track-averaged LET. These two methods yields identical results for monoenergetic charged particles [Citation7,Citation8], but large differences between the two methods appear for fragmented beams [Citation9,Citation10]. Not all publications have stated which way they have calculated their LET values, but amongst the ones that have, all track-averaged LET values came from monoenergetic beams. A few studies have stated their LET differently, e.g. Todd (1967) [Citation11] where the given LET is stopping power in tissue, and Folkard (1996) [Citation12] where LET is volume averaged. In some papers, a dose response curve was shown, but the RBE values were not reported. In that case, where it was possible, we calculated the RBE by reading of the dose values at the isoeffect dose of the ions and the reference radiation using WinDIG2.5 (www.unige.ch/sciences/chifi/cpb/windig.html). Thirteen papers used x-rays while three papers used 60Co as reference beams. All obtained data points are from experiments using normoxic conditions. As the compiled data is from different studies, some experimental conditions varied. For publications on V79 cells, there were different V79 sublines used (V79-4, V79-379A and V79-753B).
We found 228 published RBE in vitro data points from 26 publications on inactivation of V79, CHO and T1 cells irradiated with various particle types. For V79, data points were collected from 20 publications with 139 RBE values from 11 different particle types. For CHO there were four publications with 37 RBE values from four different particle types. For the T1 cells there were four publications with 54 RBE values for eight different particle types. More papers reported several cell types and particle types.
Regression analysis where performed on the RBE-LET plots (LET ≤ 100 keV/μm) using the statistical package SPSS (SPSS Inc., Chicago, IL). Furthermore slopes and intercepts were for each cell line compared between plots of carbon ions and plots of all particles following [Citation13].
All extracted data are shown in a supplementary table available at http//www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.582518.
Results
RBE against LET curves has been studied in a comparative approach with respect to the different ion types. As RBE is highly dependent on cell type and on endpoint, we discriminated the RBE-LET relationship for the three investigated cell lines and same endpoint. The three cell lines V79, CHO and T1 were chosen based on the level of comparable published data.
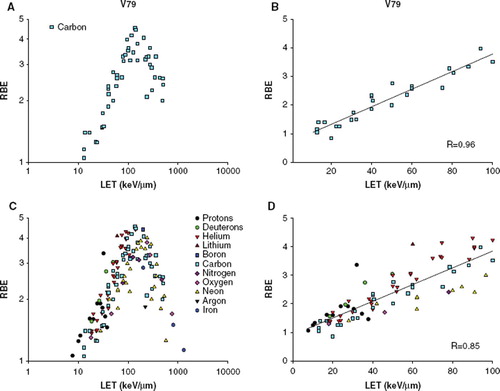
For V79 cells, 49 RBE values for carbon ions from eight different publications [Citation3,Citation5,Citation14–19] were plotted as a function of the reported LET value (). As the different publications have calculated their RBE values differently in regards to whether it is a mean value from more experiments, and how a standard deviation is derived (if any is reported), we did not include standard errors. RBE was plotted on a logarithmic axis, as the relative standard deviation appeared to be constant at both high and low RBE values (). To compare to the RBE-LET relationship from various particles, 139 RBE values from 11 different particle types [Citation12,Citation20–30], in the range 7.7 to 2106 keV/μm, were plotted similar to the carbon data (). There is a high variation in the number of data points for each particle type, for some particles, only one or two data points have been reported (as argon, boron, lithium or nitrogen), whereas other particle types were much better represented. Also, particles as protons are restricted to a maximum LET of 37.8 keV/μm and iron ions have only been reported in the range from 200 to 2106 keV/μm. The general trend for the particles was that all the RBE values increased with an increase of LET, and reached a peak at ∼100–200 keV/μm. When plotted together, all RBE-LET data points demonstrate surprising agreement within the general error band formed by the numerous data points. RBE data points against LET ≤ 100 keV/μm was plotted ( and ), to be able to analyze the RBE data on the ascending part of the curve. Regression analysis were significant for both plots (p < 0.0001 for both plots, correlation coefficients 0.96 and 0.85). The two slopes and y-intercepts were tested against each other, and were found not to be different.
Figure 1. RBE-LET plot for V79 cells A) For carbon ions. B) For carbon ions, LET below 100 keV/μm. The solid line represents the regression line (RBE = 0.709 + (0.0308 *LET)). C) For additional particle types. D) For additional particle types, LET below 100 keV/μm. The solid line represents the regression line (RBE = 0.915 + (0.0293 *LET)).
For CHO cells 17 RBE values from carbon ions from four different publications [Citation3,Citation31–33] were plotted (). The RBE values increase with increasing LET, with a peak at ∼100–200 keV/μm. When plotted together with data points from other particle types (argon: two RBE values, iron: three RBE values and neon: 15 RBE values) (), there is no difference in the course of the curve. Regression analysis on the ascending part of the curve were significant for both plots (p < 0.005 for both plots, correlation coefficients 0.96 and 0.94) ( and ). The slopes and intercept of the regression lines are tested not to be different from each other.
Figure 2. RBE-LET plot for CHO cells A) For carbon ions. B) For carbon ions, LET below 100 keV/μm. The solid line represents the regression line (RBE = 1.258 + (0.0169 *LET)). C) For additional particle types. D) For additional particle types, LET below 100 keV/μm. The solid line represents the regression line (RBE = 1.243 + (0.0160 *LET)).
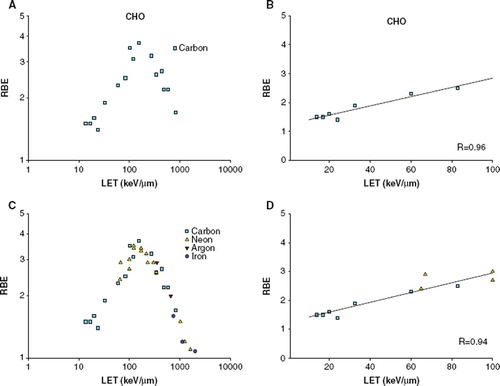
Figure 3. RBE-LET plot for T1 cells A) For carbon ions. B) For carbon ions, LET below 100 keV/μm. The solid line represents the regression line (RBE = 0.863 + (0.0245* LET)). C) For additional particle types. D) For additional particle types, LET below 100 keV/μm. The solid line represents the regression line (RBE = 0.941 + (0.0209 *LET)).
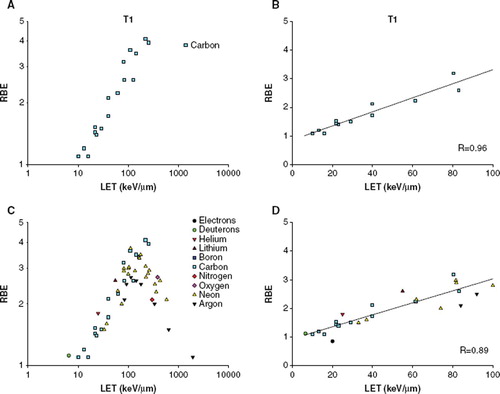
The reported data points for T1 cells include 53 RBE values from four publications [Citation5,Citation11,Citation34,Citation35]. The LET for the carbon ions range from 10–252 keV/μm. Within this range the RBE-LET curve do not reach a peak or start to decline (). The variance in the data when more particle types are included is again very limited (). Regression analysis on the ascending part of the curve were significant for both plots (p < 0.005 for both plots, correlation coefficients 0.96 and 0.89) ( and ). The slopes and intercept of the regression lines are not significantly different from each other.
Discussion
It has previously been shown that RBE, besides LET, depends on particle type. In this study, we have compared a large range of already published RBE-LET data from three different cell lines. The aim was to compare the RBE-LET relationship from different particle types, in order to visualize the magnitude of the effect of different ion-types.
As RBE is highly dependent on cell type and endpoint, we discriminated the RBE-LET relationship for the three investigated cell lines for a single endpoint (10% survival in colony formation). Data points were collected from 20, four and four publications for V79, CHO and T1, respectively, in total covering 228 RBE values from a broad range of particle species.
This review is on data from in vitro studies, and not in vivo or clinically based, due to the level of comparable data. This off course presents limitation to the study, and to the conclusions drawn thereof. The in vitro situation is very simplified compare to the much more complicated in vivo situation, which is characterized by high heterogeneity and influence of microenvironmental factors, such as hypoxia and low pH.
Previous meta-analyses have also addressed the LET dependence of RBE. Ando et al. (2009) [Citation36], have analyzed a wide range of RBE data, and demonstrated a correlation between the LET of carbon ions and RBE values from more studies. In Belloni 2002 it is concluded that the RBE against LET curves for V79 depends strongly on the type of ion [Citation37]. However, only limited data is included in this study, where it looks like a very apparent effect of the different ion types. When more data points are included to the same types of plots, it becomes quite obvious that this difference is rather small and hidden by the general errors arising from the biology.
In our study we show that when we plot data from a range of studies for a large number of different particles, all RBE-LET data points demonstrate surprising agreement within the general error band formed by the numerous data points, and display the expected RBE peak at around 100–200 keV/μm. For all three cell lines, the influence of varying the particle type on the RBE was far from obvious, compared to the general experimental noise. Therefore this supports the widespread assumption that the RBE for the same cell line and the same biologic endpoint may be assumed to be dependent on LET alone.
Yet, we will not object to the fact that there is a particle difference, which is well established. Instead, we call for a possibility of addressing it in a more quantitative way. In other words: can a single-valued LET approximately be adopted without introducing a significant error to the subsequently calculated RBE?
The motivation for this view arises from multiple contradictions: 1) When investigating the RBE as a function of LET, it is clear that even for the same particle there are two LET values which give the same RBE. However, given the energy spectrum of high-LET ions applied in radiotherapy, the one of the two LET values are found at the distal edge of the Bragg-peak, where particle suddenly come to rest. In practice in such a point a wide range of LET values will be found, which will dilute the average (either dose or track average) in this point; and 2) Different ions at different energy may have equal LET but different RBE. Again these iso-LET situations may occur either in the low or high energy regime. One could assume from premise 1) that the low-LET radiation is unimportant. The question remains then, how large is the resulting uncertainty from an iso-LET = iso-RBE assumption? The nature of the answer to this question is depending on the context wherein the assumption is applied, and remains to be investigated. Such a formalism will possibly also be very relevant for detector response models, and could enable new ways of measuring LET, which will become relevant if new treatment strategies such as LET-painting is realized [Citation38].
Here, the present data demonstrates that the particle dependence of LET is very small, and suggests that a general model for the RBE-LET relationship may be formulated.
http://www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.582518
Download PDF (64.1 KB)Acknowledgements
This work was supported by ULICE – Union of Light Ion Centres in Europe, the Danish Cancer Society, and CIRRO – The Lundbeck Foundation Centre for Interventional Research in Radiation Oncology.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Goodhead DT. Mechanisms for the biological effectiveness of high-LET radiations. J Radiat Res (Tokyo) 1999;40(Suppl):1–13.
- Jakel O. The relative biological effectiveness of proton and ion beams. Z Med Phys 2008;18:276–85.
- Weyrather WK, Ritter S, Scholz M, Kraft G. RBE for carbon track-segment irradiation in cell lines of differing repair capacity. Int J Radiat Biol 1999;75:1357–64.
- Weyrather WK, Kraft G. RBE of carbon ions: Experimental data and the strategy of RBE calculation for treatment planning. Radiother Oncol 2004;73(Suppl 2):S161–9.
- Furusawa Y, Fukutsu K, Aoki M, Itsukaichi H, Eguchi-Kasai K, Ohara H, . Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He-, (12)C- and (20)Ne-ion beams. Radiat Res 2000;154:485–96.
- Butts JJ, Katz R. Theory of RBE for heavy ion bombardment of dry enzymes and viruses. Radiat Res 1967;30:855–71.
- International Commission on Radiation Units and Measurements (ICRU). Linear Energy Transfer. ICRU report 16:1970.
- Hall EJ, Giaccia A. Radiobiology for the radiologist. 6th Philadelphia: Lippincott Williams & Wilkins; 2006.
- Durante M, George K, Gialanella G, Grossi G, La TC, Manti L, . Cytogenetic effects of high-energy iron ions: Dependence on shielding thickness and material. Radiat Res 2005; 164(4 Pt 2):571–6.
- Bassler N, Holzscheiter M. Calculated LET spectrum from antiproton beams stopping in water. Acta Oncol 2009;48: 223–6.
- Todd P. Heavy-ion irradiation of cultured human cells. Radiat Res Suppl 1967;7:196–207.
- Folkard M, Prise KM, Vojnovic B, Newman HC, Roper MJ, Michael BD. Inactivation of V79 cells by low-energy protons, deuterons and helium-3 ions. Int J Radiat Biol 1996;69:729–38.
- Zar HJ. Comparing simple linear regression equations. Biostatistical analysis. Englewood Cliffs, NJ: Prentice-Hall International; 1974. 353–61.
- Aoki M, Furusawa Y, Yamada T. LET dependency of heavy-ion induced apoptosis in V79 cells. J Radiat Res (Tokyo) 2000; 41:163–75.
- Belli M, Bettega D, Calzolari P, Cherubini R, Cuttone G, Durante M, . Effectiveness of monoenergetic and spread-out bragg peak carbon-ions for inactivation of various normal and tumour human cell lines. J Radiat Res (Tokyo) 2008;49: 597–607.
- Chapman JD, Doern SD, Reuvers AP, Gillespie CJ, Chatterjee A, Blakely EA, . Radioprotection by DMSO of mammalian cells exposed to x-rays and to heavy charged-particle beams. Radiat Environ Biophys 1979;16:29–41.
- Hirayama R, Ito A, Tomita M, Tsukada T, Yatagai F, Noguchi M, . Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiat Res 2009;171:212–8.
- Pathak R, Sarma A, Sengupta B, Dey SK, Khuda-Bukhsh AR. Response to high LET radiation 12C (LET, 295 keV/microm) in M5 cells, a radio resistant cell strain derived from Chinese hamster V79 cells. Int J Radiat Biol 2007;83:53–63.
- Zhou G, Kawata T, Furusawa Y, Aoki M, Hirayama R, Ando K, . Protective effects of melatonin against low- and high-LET irradiation. J Radiat Res (Tokyo) 2006;47:175–81.
- Belli M, Cherubini R, Finotto S, Moschini G, Sapora O, Simone G, . RBE-LET relationship for the survival of V79 cells irradiated with low energy protons. Int J Radiat Biol 1989;55:93–104.
- Belli M, Cera F, Cherubini R, Haque AM, Ianzini F, Moschini G, . Inactivation and mutation induction in V79 cells by low energy protons: Re-evaluation of the results at the LNL facility. Int J Radiat Biol 1993;63:331–7.
- Belli M, Cera F, Cherubini R, Dalla VM, Haque AM, Ianzini F, . RBE-LET relationships for cell inactivation and mutation induced by low energy protons in V79 cells: Further results at the LNL facility. Int J Radiat Biol 1998;74:501–9.
- Cox R, Thacker J, Goodhead DT, Munson RJ. Mutation and inactivation of mammalian cells by various ionising radiations. Nature 1977;267(5610):425–7.
- Folkard M, Prise KM, Vojnovic B, Davies S, Roper MJ, Michael BD. The irradiation of V79 mammalian cells by protons with energies below 2 MeV. Part I: Experimental arrangement and measurements of cell survival. Int J Radiat Biol 1989;56:221–37.
- Pathak R, Dey SK, Sarma A, Khuda-Bukhsh AR. Genotoxic effects in M5 cells and Chinese hamster V79 cells after exposure to 7Li-beam (LET = 60 keV/microm) and correlation of their survival dynamics to nuclear damages and cell death. Mutat Res 2007;628:56–66.
- Pathak R, Dey SK, Sarma A, Khuda-Bukhsh AR. Cell killing, nuclear damage and apoptosis in Chinese hamster V79 cells after irradiation with heavy-ion beams of (16)O, (12)C and (7)Li. Mutat Res 2007;632:58–68.
- Stoll U, Schmidt A, Schneider E, Kiefer J. Killing and mutation of Chinese hamster V79 cells exposed to accelerated oxygen and neon ions. Radiat Res 1995;142:288–94.
- Raju MR, Eisen Y, Carpenter S, Inkret WC. Radiobiology of alpha particles. III. Cell inactivation by alpha-particle traversals of the cell nucleus. Radiat Res 1991;128:204–9.
- Stenerlow B, Pettersson OA, Essand M, Blomquist E, Carlsson J. Irregular variations in radiation sensitivity when the linear energy transfer is increased. Radiother Oncol 1995; 36:133–42.
- Tilly N, Brahme A, Carlsson J, Glimelius B. Comparison of cell survival models for mixed LET radiation. Int J Radiat Biol 1999;75:233–43.
- Czub J, Banas D, Blaszczyk A, Braziewicz J, Buraczewska I, Choinski J, . Biological effectiveness of (12)C and (20)Ne ions with very high LET. Int J Radiat Biol 2008;84: 821–9.
- Czub J, Banas D, Blaszczyk A, Braziewicz J, Buraczewska I, Choinski J, . Cell survival and chromosomal aberrations in CHO-K1 cells irradiated by carbon ions. Appl Radiat Isot 2009;67:447–53.
- Sasaki H, Yatagai F, Kanai T, Furusawa Y, Hanaoka F, Zhu WG, . Dependence of induction of interphase death of Chinese hamster ovary cells exposed to accelerated heavy ions on linear energy transfer. Radiat Res 1997;148:449–54.
- Barendsen GW, Beusker TL, Vergroesen AJ, Budke L. Effects of different radiations on human cells in tissue culture. II. Biological experiments. Radiat Res 1960;13:841–9.
- Blakely EA, Tobias CA, Yang TCH, Smith KC, Lyman JT. Inactivation of human kidney cells by high-energy monoenergetic heavy-ion beams. Radiat Res 1979;80:122–60.
- Ando K, Kase Y. Biological characteristics of carbon-ion therapy. Int J Radiat Biol 2009;85:715–28.
- Belloni F, Bettega D, Calzolari P, Cherubini R, Massariello P, Tallone L. Inactivation cross sections for mammalian cells exposed to charged particles: A phenomenological approach. Radiat Prot Dosimetry 2002;99:199–202.
- Bassler N, Jakel O, Sondergaard CS, Petersen JB. Dose- and LET-painting with particle therapy. Acta Oncol 2010;49: 1170–6.