Abstract
Background. Radiotherapy (RT) for abdominal and pelvic malignancies often causes severe small bowel toxicity. Citrulline concentrations are known to decrease with intestinal failure. We thus evaluated the feasibility of plasma citrulline levels in predicting radiation-induced intestinal toxicity. Material and methods. Fifty-three patients (36 prostate cancer, 17 endometrial cancer) who received 45 Gy pelvic RT using conventional fractionation were prospectively evaluated. Patients with prostate cancer received an additional 25–30.6 Gy conformal boost. Plasma citrulline levels were assessed on day 0, mid- (week 3) and post-RT (week 8), and four months post-RT. Dose-volume histogram, citrulline concentration changes, and weekly intestinal toxicity scores were analyzed. Results. Mean age was 63 years (range: 43–81 years) and mean baseline citrulline concentration was 38.0 ± 10.1 μmol/l. Citrulline concentrations were significantly reduced at week 3 (27.4 ± 5.9 μmol/l; p < 0.0001), treatment end (29.9 ± 8.8 μmol/l; p < 0.0001), and four months post-treatment (34.3 ± 12.1; p = 0.01). The following factor pairs were significantly positively correlated: Citrulline concentration/mean bowel dose during, end of treatment, and four months post-RT; dose-volume parameters/citrulline change groups; cumulative mean radiation dose/intestinal toxicity at end and four months post-RT; citrulline changes/intestinal toxicity during and end of RT. Citrulline concentration changes significantly differed during treatment according to RTOG intestinal toxicity grades (p < 0.0001). Although the citrulline changes differed significantly within RTOG intestinal toxicity grades (p = 0.003), the difference between Grade 0 and Grade 1 did not differ significantly at the end of the treatment. At four months after RT, no significant differences were apparent. Conclusion. Citrulline-based assessment scores are objective and should be considered in measuring radiation-induced intestinal toxicity.
Radiotherapy (RT) plays an important role in the treatment of abdominopelvic malignancies. The small bowel is usually the dose-limiting organ during treatment of abdominal and pelvic malignancies. Severe toxicity in this organ sometimes requires treatment interruption, which is an established detrimental factor for treatment outcome [Citation1]. The actuarial five-year rates of severe small bowel injury, such as fistulas and strictures, are usually less than 5% after conventional doses of 45–50 Gy [Citation2]. However, persisting functional changes causing chronic diarrhea, malabsorption, and other symptoms associated with small bowel injury have been reported in 40–50% of patients treated with RT at conventional doses [Citation2]. Several functional changes have been reported to be associated with an impaired absorptive capacity due to loss of radiation-induced epithelial cells. Thus, assessing and monitoring mucosal damage, as well as early detection of mucositis, are required for effective toxicity management.
Unfortunately, no simple and objective biologic marker has been described to date that is sensitive and specific for radiation-induced small bowel mucosal atrophy. Plasma citrulline, a nitrogen end product of small bowel enterocyte glutamine metabolism, has been used as a marker for evaluating functional small bowel enterocyte mass [Citation3,Citation4]. Injury to the small intestine can be measured by monitoring the decline in circulating citrulline. Because plasma citrulline concentrations are highly dependent upon intestinal cell mass, low citrulline concentrations are associated with intestinal failure independent of underlying causes.
The clinical use of citrulline as a biomarker for intestinal toxicity related to chemotherapy regimens, surgery, or other conditions related to intestines have been widely studied [Citation3,Citation5–8]. However, the clinical use of citrulline for radiation-induced intestinal toxicity has not yet been analyzed in detail [Citation9]. Using small bowel dosimetric and volumetric parameters, as well as clinical symptoms, we evaluated the feasibility of citrulline levels in predicting radiation-induced intestinal toxicity in homogenous patient groups treated with RT.
Material and methods
Patient characteristics
This prospective study, conducted between November 2008 and May 2010, included 53 patients diagnosed with either prostate cancer or endometrial cancer, and treated with pelvic conformal RT. Of the 53 patients, 37 patients had prostate cancer and 17 had endometrial cancer. Exclusion criteria included treatment with chemotherapy at any time, impaired renal function (glomerular filtration rate [GFR] <60 ml/min) less than four weeks before the start of treatment, or previous abdominal and pelvic region irradiation. Agents that might ameliorate mucositis were not administered during RT. In all patients, the assessment of clinical toxicity and the acquisition of blood samples for plasma citrulline measurements were performed on the same day. All patients provided written informed consent and the study design was approved by the institutional ethics committee.
RT planning
A planning computed tomography (CT)-scan with a slice thickness of 2.5 mm was performed with patients in a supine position with their feet fixed in a commercially-available knee support device. Patients were instructed to drink 500 ml of oral contrast solution 30 min before the scan. The intestine was contoured up to the level of L2 to make intestinal volumes identical in all patients. All contouring was performed by one author (A.K.) and checked by another (C.O.); both authors had no knowledge of citrulline measurement results or dose–volume histograms (DVH).
Following DVH analysis, the bowel volume that received a dose between 5 Gy and 55 Gy at 5 Gy intervals (V5Gy–V55Gy), and the bowel that received at least 50% of the prescribed radiation dose (V50%) were calculated. For statistical analysis, volumetric parameters were expressed as the percentage of total volume. Volumetric and dosimetric parameters were correlated with the Radiation Therapy Oncology Group (RTOG) toxicity score for acute radiation-related intestinal toxicity and with the plasma citrulline concentration at corresponding time points.
RT
All patients were treated with conventional fractionation at 1.8–2 Gy fraction doses using 18 MV photon energies (Varian DHX 3323; Varian Medical Systems, PaloAlto, CA). Patients with prostate cancer were treated definitively without surgery, while patients with endometrial cancer were adjuvantly treated after radical hysterectomy with pelvic lymphadenectomy. The pelvic field of patients with prostate cancer was treated with 45 Gy total dose, which included the prostate, seminal vesicles, and lymphatics at initial phase. In the second phase of treatment, a median 25.6 Gy (range 24–30.6 Gy) conformal boost to the prostate and seminal vesicles was administered. For 17 patients with endometrial cancer, RT was combined with three high-dose rate brachytherapy applications with a total prescribed dose of 4 Gy for 14 patients and 5 Gy for three patients.
Plasma citrulline measurements
Plasma citrulline concentrations were assessed approximately 15 min before irradiation. Before the blood sample was taken, patients fasted overnight and were instructed to lie down for 15 min in a hospital bed in order to avoid physical exertion. An antecubitalvein was used to sample 1.5 ml blood, which was collected in a heparinized cup and stored on ice. Plasma was then obtained through whole blood centrifugation at 10 000 × g for 10 min at 4°C. For amino acid determination, 250 μl of plasma was first deproteinized by adding the plasma to 22 mg dry 5-sulfosalicylic acid. The plasma mixture was then vortexed, frozen in liquid nitrogen, and stored at 280°C until further processing. Plasma citrulline concentration (μmol/l) was measured with high-performance liquid chromatography [Citation4]. Baseline concentrations were first assessed at day 0, just prior to first RT treatment. Subsequently, plasmacitrulline concentrations were measured at the middle (week 3) and end of treatment (week 8), as well as four months after RT. Rather than assessing weekly citrulline levels during RT, citrulline was measured in blood samples taken during week 3 of treatment, which corresponded to the previously determined maximum period of decline of this protein [Citation9].
Clinical parameters
Same physician examined each patient weekly during RT, as well as at four weeks and four months after completion of RT to assess treatment-related morbidity. During each visit, clinical symptoms attributable to intestinal toxicity were recorded using the RTOG scoring criteria for acute radiation morbidity of the lower gastrointestinal tract [Citation10]. The intestinal toxicity scores were also assessed at the month 4 visit. To exclude the possibility of intestinal infection, a microbiological assessment was performed if and when diarrhea occurred. For statistical analysis, the RTOG scores at each time point were used, as was the percentage of total RT treatment time that a patient was free of symptoms.
Statistical analysis
The citrulline concentrations measured at various time points were compared using a paired-samples t-test. To test correlations between citrulline concentration, dose-volume, and clinical parameters, Pearson correlation and one-way analysis of variance (ANOVA) used with Tukey's post-hoc testing were used, where when appropriate. Correlations were considered significant at p ≤ 0.01. Pearson correlation factors of ≥0.7 were considered a good correlation between datasets. A correlation was considered moderate at 0.5–0.7, and poor at < 0.5. One-way ANOVA was used to test a dose and volume relationship for serum citrulline and clinical toxicity, respectively. The changes in serum citrulline concentrations at three different time points were compared to the baseline concentration, which was measured in a sample taken before RT, middle of treatment (week 3), end of treatment (week 8), and four months after treatment. Patients were assigned to one of three groups according to the change in plasma citrulline concentrations: 0–20% change, 21–40% change and > 40% change. DVH analysis was correlated according to the citrulline change groups.
Results
All patients completed the treatment protocol as planned. The mean age of all patients was 63 years (range: 43–81 years). Thirty-six patients (68%) were male with a diagnosis of prostate cancer and 17 patients (32%) were female with a diagnosis of endometrial cancer. The mean age of male patients was 66 years (range 54–79 years) and the mean age of female patients was 58 years (range 43–81 years). There were no significant dose-volume changes between patients with prostate cancer and patients with endometrial cancer, except for Dmax, V40Gy, and V45Gy.Dmax was significantly higher in patients with prostate cancer (63.7 ± 12.6 Gy vs. 49.1 ± 4.2 Gy; p = 0.001), which was due to higher prescribed RT doses for prostate cancer treatment. However V40Gy and V45Gy were significantly higher in patients with endometrial cancer compared to prostate cancer patients (19.6 ± 10.6% vs. 16.2 ± 9.3%; p = 0.03 and 14.1 ± 8.7% vs. 11.2 ± 8.4%; p = 0.03).
Citrulline concentration
The mean citrulline concentration at baseline for all patients was 38.0 ± 10.1 μmol/l. The mean citrulline concentration was significantly reduced at week 3 of treatment (27.4 ± 5.9 μmol/l; p < 0.0001), at the end of the treatment (week 8; 29.9 ± 8.8 μmol/l; p < 0.0001), and at four months after treatment (34.3 ± 12.1μmol/l; p = 0.01). During RT, citrulline concentrations were reduced in 51 of the total 53 patients (mean decrease: 27%, range: 2–69%). In the remaining two patients, the mean increase in citrulline concentration was 7% (range: 3–10%). At the end of RT, citrulline concentrations were significantly reduced in 43 of the 53 patients (mean decrease: 26%, range: 3–71%), significantly increased in nine patients (mean increase: 11%, range: 4–27%), and unchanged in one patient. At month 4 after RT, citrulline concentrations were reduced in 37 patients (mean decrease: 21%, range: 2–64%) and increased in 16 patients (mean increase: 21%, range: 3–47%).
There was no significant difference in baseline citrulline levels between patients with endometrial cancer compared to those with prostate cancer. But citrulline levels were significantly higher at week 3 (p = 0.001), end of treatment (week 8) (p < 0.001), and month 4 (p = 0.03), in patients with prostate cancer compared to patients with endometrial cancer (). However, the difference in citrulline concentration change was significantly higher in endometrial cancer patients at the end of treatment (p = 0.01). Although the citrulline concentration change was nearly significantly higher in patients with endometrium cancer compared to patients with prostate cancer at week 3 (p = 0.09), the significance was lost at month 4 (p = 0.2).
Table I. Mean ± S.D. citrulline levels and concentration changes according to diagnosis groups.
Citrulline and dose-volume histogram relationship
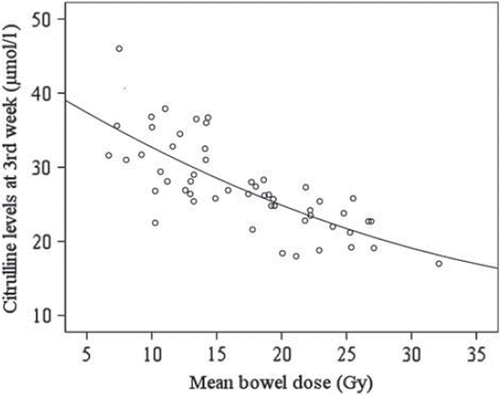
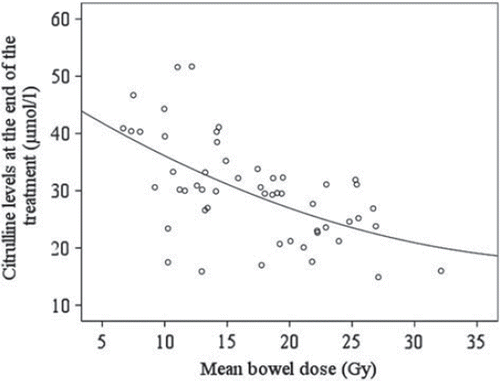
During RT, a significantly good correlation existed between citrulline concentrations and total mean bowel dose (Pearson r =−0.75; p < 0.001) (). However, the correlation between citrulline concentrations and total mean bowel dose was moderate at the end of RT (Pearson r = –0.58; p < 0.001; ), and poor at four months after treatment (Pearson r = –0.34; p = 0.01).
Figure 2. Correlation between mean bowel dose and plasma citrullin level measured at the end (week 8) of RT.
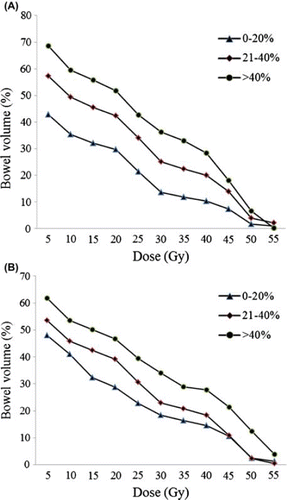
The three citrulline concentration change groups, (1) < 20%, (2) 21–40%, and (3) > 40%, were associated with the following mean bowel doses: 14.7 ± 5.1 Gy, 19.0 ± 5.7 Gy, and 19.6 ± 7.7 Gy, respectively (p = 0.05). As determined by dose-volume analysis, V5Gy–V45Gy values measured during RT were significantly different between Groups 1, 2, 3 (p < 0.001 for V5–V40Gy between each successive groups, and p = 0.007 for V45Gy only between Group 1 and 2) (). In contrast, V50Gy and V55Gy values did not differ significantly during RT. For citrulline change groups measured at the end of treatment, V15Gy– V40Gy values differed significantly between groups and within groups (p = 0.01, 0.02, 0.03, 0.02, 0.04, and 0.02 for V15–V40Gy between each groups) (). V45Gy,V50Gy, and V55Gy were not significantly different between the three groups; however, V45Gy and V50Gy values were significantly different between Group 1 vs. Group 3 (p = 0.001) and Group 2 vs. Group 3 (p = 0.001).
Intestinal toxicity
During RT, intestinal toxicity occurred at the following severity grades: 13 patients had Grade 1 (25%), 30 patients (57%) had Grade 2 and 10 patients (19%) had no intestinal toxicity. The cumulative mean radiation dose was significantly positively correlated with intestinal toxicity during RT (week 3) and at the end of RT (week 8) (Pearson r = 0.55 and 0.62; p = 0.008 and p = 0.01, respectively). Toxicity, which was assessed weekly, was significantly associated with successive bowel dose levels of 5–45 Gy during RT and 5–40 Gy at the end of RT.
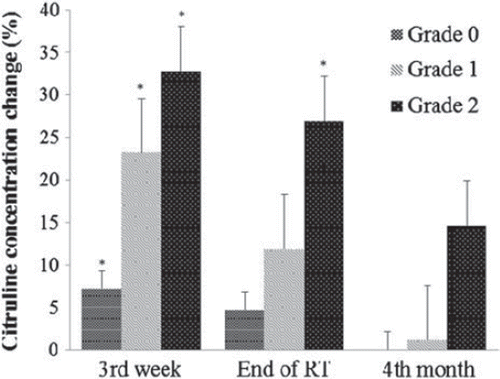
Citrulline concentrations were significantly positively correlated with intestinal toxicity during RT (week 3), at the end of RT (week 8), and four months after RT (Pearson r =−0.66, −0.48 and 0.40; p = 0.0001,p = 0.0002, and p = 0.003, respectively). Citrulline concentration changes significantly differed during treatment according to RTOG intestinal toxicity grades (p < 0.0001; ). Although the citrulline changes differed significantly within RTOG intestinal toxicity grades (p = 0.003), the difference between Grade 0 and Grade 1 did not differ significantly at the end of the treatment. However, the difference between Grade 1 vs. Grade 2 and Grade 0 vs. Grade 2 did differ significantly (p = 0.03 and p < 0.0001). At four months after RT, no significant differences were apparent between the changes in citrulline concentration and RTOG toxicity scores. Additionally, there were no significant differences in intestinal toxicity scores between patients with prostate cancer and endometrial cancer during RT, at the end of RT, and four months after RT.
Discussion
In this prospective study, the role of citrulline in predicting radiation-induced intestinal toxicity was assessed both clinically and with dosimetric parameters in patients with endometrial and prostate cancer. The results of this study demonstrated clearly that patients with higher intestinal dose and RTOG toxicity score experienced a significantly higher decline in citrulline levels.
Intestinal toxicity is a significant clinical problem in patients receiving RT to the abdomen or pelvis. Because the small intestine is one of the most radiosensitive organs, abdominopelvic RT may induce inflammation in the small intestine resulting in submucosal edema, hyperemia, and infiltration of the lamina propria by activated inflammatory cells [Citation11]. The structural changes in the intestine caused by RT are attributable to alterations in various extracellular mediators and their intracellular messengers [Citation12]. These RT-induced structural changes may cause diarrhea, malabsorption, and weight loss. Various attempts have been made to decrease intestinal toxicity in patients being treated with RT, such as using more sophisticated techniques, including three-dimensional (3D) conformal RT, intensity modulated RT, and tomotherapy that enables more precise treatment planning with better sparing of the normal tissues. However, intestinal toxicity still remains the dose-limiting toxicity for abdominal and pelvic RT.
At present, there is no ‘gold standard’ measure for toxicity that accurately assesses the extent of damage in the gut with which biological marker data can be compared. Currently, the National Cancer Institute-common terminology criteria for adverse events scale, version 3.0,and RTOG toxicity score are considered the best scale for intestinal mucositis and is designed based on signs and symptoms related to gastrointestinal changes [Citation13]. In clinical practice RTOG toxicity scoring system for assessing the intestinal toxicity has been frequently used, as was in this study. However, these scoring systems have several drawbacks, including a lack of reliability and validation Actually, the scoring system used for assessing intestinal toxicity is neither specific nor objective, and depends entirely on patient description and physician assessment. A clinician's assessment of toxicity or a patient-completed symptom questionnaire does not lead to an objective understanding of the mechanisms that cause symptoms and thus impairs classification of the damage incurred. Furthermore, the signs and symptoms are influenced by the use of anti-emetics and opioids for analgesia, which induce constipation [Citation14]. Although RTOG scoring system is probably among the best tool we have, an objective scoring system or biological marker is needed for the appropriate evaluation of intestinal toxicity. An assessment score based on citrulline may be an objective marker, but not clearly validated yet. Also the concentration of this protein depends on the volume of intestine that has been injured by surgery, chemotherapy, RT or other causes, the specificity and sensitivity of this marker for predicting the intestinal toxicity is not clearly well-defined. For measuring intestinal mucositis, a citrulline-based scoring method meets the criteria proposed by the Multinational Association of Supportive Care in Cancer and the International Society for Oral Oncology more accurately than any other scoring system [Citation15]. This current study contributes to the level of confidence in using citrulline as a predictor for radiation-induced intestinal toxicity.
Citrulline is a nitrogen end product of small bowel enterocyte glutamine metabolism and accounts for almost 30% of metabolized glutamine nitrogen in small intestine [Citation16]. Small-intestinal enterocytes contain specific mitochondrial enzymes involved in citrulline production, but lack the cytosolic enzymes necessary for its conversion to arginine [Citation17]. This unique enzymatic profile and the fact that citrulline is not metabolized by the liver means that the small bowel is the principal source of circulating citrulline [Citation18]. The relationship between plasma citrulline concentration and epithelial cell mass has been demonstrated previously [Citation18]. Recently, several clinical studies involving patients with short-bowel syndrome [Citation3], villous atrophy-associated intestinal disease [Citation19], small intestine transplantation [Citation20], and chemotherapy-induced mucosal enteropathy [Citation6,Citation7,Citation21] have shown that citrulline levels positively correlate with overall small bowel function. However, few studies have investigated the relationship between citrulline levels and radiation-induced enteropathy [Citation4,Citation9]. Lutgens et al. first analyzed the use of citrulline for radiation-induced small bowel atrophy in a feasibility study involving 23 patients. In this study, the authors evaluated a group of patients that was more heterogeneous than the present study, with more variability in diagnoses, treatment modalities, and RT fields. In this current study, the patient number is doubled, and more importantly we evaluated a relatively homogenous group of patients with similar treatment modalities: One group consisted of patients with prostate cancer treated with RT definitively; the other group of patients had endometrial cancer and was treated with pelvic RT postoperatively. Additionally, because the detrimental effect of chemotherapy on citrulline levels has been clearly demonstrated [Citation6,Citation21], no patients in this study received chemotherapy neoadjuvantly, concurrently, or adjuvantly.
Radiation-induced intestinal toxicity is clearly associated with small bowel dose and volume of small bowel irradiated [Citation2,Citation22–24]. We identified a significant positive correlation between intestinal dose and volume for radiation-induced toxicity both during RT and at the end of the treatment. Additionally, a significant positive correlation existed between dose-volume changes and intestinal toxicity scores, in concordance with a previously reported study [Citation4] using dose levels of 5–40 Gy. However, we found no significant dose-volume correlation for late sequela evaluated at month 4, which may be due to limited follow-up time.
Recently, Crenn et al. [Citation3] identified a correlation between plasma citrulline concentration with histologically-graded villous atrophy in 42 patients with celiac villous atrophy disease and 10 patients with the non-celiac form of this disease. The authors identified a threshold value of 10 μmol/l (25% of the mean normal baseline value) as predictive of severe and extensive villous atrophy and 20 μmol/l as predictive of severe villous atrophy, regardless of extent. Lutgens et al. [Citation9] applied these threshold levels to patients treated with RT and found a positive correlation between citrulline concentration and intestinal dose and toxicity. However, the treatment fields used in Lutgens’ study were variable and somewhat larger than those of the present study. As a result, greater intestine volumes were irradiated, which resulted in very low plasma citrulline levels, some of which were < 10 μmol/l. In this current study, only pelvic fields were treated, which resulted in less intestinal volume irradiation. Because the intestinal effects of radiation are temporary and the fields used in this study were less than those in previous studies, the plasma citrulline concentrations during and after RT were not very low. Additionally, there is no clearly defined threshold to indicate intestinal toxicity after RT. For these reasons, rather than using predefined threshold levels, we chose to use citrulline change ratios during, at the end, and after RT. In the current study, we identified a significant positive correlation between plasma citrulline concentration and bowel doses both during RT () and at the end of RT (). Similarly, the changes in citrulline levels during RT and at the end of RT significantly differed as a function of bowel dose-volume parameters (Figures 3A and B).
Plasma citrulline and RTOG toxicity score were used as end points for radiation-induced small bowel toxicity in this study. The cumulative intestine dose and citrulline levels were significantly correlated with intestinal toxicity scores (). Overall, citrullinemia correlates poorly with the severity of clinical symptoms as graded by the RTOG acute toxicity score used in this study. However, a change in plasma citrulline concentration was detected in patients that had no intestinal toxicity either during or after RT. These results may be explained in part by the fact that radiation-induced epithelial cell loss, which is typically measured by the plasma citrulline level, is merely one of several pathophysiological mechanisms underlying clinical symptoms. Additionally, the fact that clinical toxicity scores are subjective in nature may further contribute to this poor correlation. In contrast, a measure of toxicity based on plasma citrulline concentrations is more objective.
Surgical procedures may sometimes intensify the incidence of intestinal toxicity after RT [Citation25]. In order to evaluate the effect of surgery on intestinal toxicity and citrulline concentrations, we assessed only two groups: one treated with surgery, and the other without. We determined that both citrulline levels and citrulline concentration changes differed significantly in patients with endometrial cancer, which may be attributed to the significantly higher V40Gy and V45Gy levels measured in these patients. In fact, the procedure involved surgery and lymph node dissection only at pelvic area. Our data show no correlation between baseline citrulline concentration and body mass index, surgical status, or primary malignancy.
The clinical use of a serum marker as a predictor of intestinal toxicity is associated with certain complications. The serum levels of these markers may be disturbed because of various factors. Of these potential factors, intestinal infection is the most common and important reason why citrulline concentrations may be altered [Citation8]. So as not to underestimate the detrimental effects of intestinal infection, we performed microbiological evaluation of fecal content and confirmed the absence of intestinal infection during RT. Another difficulty in assessing correlations between a serum marker and dose-volume parameters is the delineation technique of the intestines. To compute bowel DVH, we used planning CT with the patient in the treatment position, an empty rectum, and a comfortably full bladder, as was previously mentioned [Citation23]. Although the intestinal contents and bowel movements could potentially change the volume irradiated, we and others have accepted this method as reliable in estimating the proportion of functional small bowel receiving a specified radiation dose by others [Citation4,Citation23].
Conclusion
A citrulline-based assessment of radiation-induced intestinal toxicity appears to be an objective method that makes it an alternative for measuring and monitoring intestinal toxicity after RT, especially as currently available tests for intestinal radiation damage are not yet suitable. Monitoring citrulline changes during and after RT is likely a feasible method for predicting radiation-induced intestinal toxicity in cases for which dose-volumetric and clinical correlation are established. However this method needs to be validated for further large scale or randomized studies for assessing the reliability and reproducibility.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Atahan IL, Onal C, Ozyar E, Yiliz F, Selek U, Kose F. Long-term outcome and prognostic factors in patients with cervical carcinoma: A retrospective study. Int J Gynecol Cancer 2007;17:833–42.
- Letschert JG, Lebesque JV, Aleman BM, Bosset JF, Horiot JC, Bartelink H, . The volume effect in radiation-related late small bowel complications: Results of a clinical study of the EORTC Radiotherapy Cooperative Group in patients treated for rectal carcinoma. Radiother Oncol 1994;32: 116–23.
- Crenn P, Vahedi K, Lavergne-Slove A, Cynober L, Matuchansky C, Messing B. Plasma citrulline: A marker of enterocyte mass in villous atrophy-associated small bowel disease. Gastroenterology 2003;124:1210–9.
- Lutgens LC, Deutz NE, Gueulette J, Cleutjens JP, Berger MP, Wouters BG, . Citrulline: A physiologic marker enabling quantitation and monitoring of epithelial radiation-induced small bowel damage. Int J Radiat Oncol Biol Phys 2003;57:1067–74.
- Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr 2008;27:328–39.
- Herbers AH, Feuth T, Donnelly JP, Blijlevens NM. Citrulline-based assessment score: First choice for measuring and monitoring intestinal failure after high-dose chemotherapy. Ann Oncol 2010;21:1706–11.
- Lutgens LC, Blijlevens NM, Deutz NE, Donnelly JP, Lambin P, de Pauw BE. Monitoring myeloablative therapy-induced small bowel toxicity by serum citrulline concentration: A comparison with sugar permeability tests. Cancer 2005;103: 191–9.
- Packey CD, Ciorba MA. Microbial influences on the small intestinal response to radiation injury. Curr Opin Gastroenterol 2010;26:88–94.
- Lutgens LC, Deutz N, Granzier-Peeters M, Beets-Tan R, De Ruysscher D, Gueulette J, . Plasma citrulline concentration: A surrogate end point for radiation-induced mucosal atrophy of the small bowel. A feasibility study in 23 patients. Int J Radiat Oncol Biol Phys 2004;60:275–85.
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341–6.
- Onal C, Kayaselcuk F, Topkan E, Yavuz M, Bacanli D, Yavuz A. Protective effects of melatonin and octreotide against radiation-induced intestinal injury. Dig Dis Sci 2011; 56:359–67.
- Giris M, Erbil Y, Oztezcan S, Olgac V, Barbaros U, Deveci U, . The effect of heme oxygenase-1 induction by glutamine on radiation-induced intestinal damage: The effect of heme oxygenase-1 on radiation enteritis. Am J Surg 2006;191:503–9.
- Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Bethesda, MD: National Cancer Institute; 2006.
- Andreyev HJ. Gastrointestinal problems after pelvic radiotherapy: The past, the present and the future. Clin Oncol (R Coll Radiol) 2007;19:790–9.
- Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, . Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004; 100(9 Suppl):1995–2025.
- Windmueller HG. Glutamine utilization by the small intestine. Adv Enzymol Relat Areas Mol Biol 1982;53:201–37.
- Wu G. Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol 1997;272(6 Pt 1): G1382–90.
- Wakabayashi Y, Yamada E, Yoshida T, Takahashi N. Effect of intestinal resection and arginine-free diet on rat physiology. Am J Physiol 1995;269(2 Pt 1):G313–8.
- Ioannou HP, Fotoulaki M, Pavlitou A, Efstratiou I, Augoustides-Savvopoulou P. Plasma citrulline levels in paediatric patients with celiac disease and the effect of a gluten-free diet. Eur J Gastroenterol Hepatol 2011;23: 245–9.
- Gondolesi G, Ghirardo S, Raymond K, Hoppenhauer L, Surillo D, Rumbo C, . The value of plasma citrulline to predict mucosal injury in intestinal allografts. Am J Transplant 2006;6:2786–90.
- Derikx JP, Blijlevens NM, Donnelly JP, Fujii H, Kanda T, van Bijnen AA, . Loss of enterocyte mass is accompanied by diminished turnover of enterocytes after myeloablative therapy in haematopoietic stem-cell transplant recipients. Ann Oncol 2009;20:337–42.
- Onal C, Topkan E, Efe E, Yavuz M, Sonmez S, Yavuz A. Comparison of rectal volume definition techniques and their influence on rectal toxicity in patients with prostate cancer treated with 3D conformal radiotherapy: A dose-volume analysis. Radiat Oncol 2009;4:14.
- Baglan KL, Frazier RC, Yan D, Huang RR, Martinez AA, Robertson JM. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 2002;52:176–83.
- Gunnlaugsson A, Kjellen E, Nilsson P, Bendahl PO, Willner J, Johnsson A. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncol 2007;46:937–44.
- Kim CW, Kim JH, Yu CS, Shin US, Park JS, Jung KY, . Complications after sphincter-saving resection in rectal cancer patients according to whether chemoradiotherapy is performed before or after surgery. Int J Radiat Oncol Biol Phys 2010;78:156–63.