Abstract
The current standard treatment for lung cancer, the most common type of cancer worldwide, depends on disease stage. Surgery is the treatment of choice for early-stage tumors, but radiotherapy is a good option for those with early-stage tumors who cannot undergo surgery, and radiotherapy in conjunction with chemotherapy is the standard of care for locally advanced tumors. Although advances in photon (x-ray)-based radiotherapy involving three-dimensional conformal radiotherapy and intensity-modulated radiotherapy allow radiation doses to be escalated beyond the traditional limit of 60 Gy, this dose is not considered to be sufficient for tumor eradication. Moreover, the improvements in local control and survival conferred by concurrent chemotherapy come at the cost of considerable toxicity owing to inadvertent irradiation of surrounding normal tissues, and this toxicity often limits the radiation dose that can be delivered. Unfortunately for patients with locally advanced lung cancer, local control and survival remain poor. Attempts to improve clinical outcomes for patients with lung cancer have led to the use of charged particle therapy in an effort to exploit the physical properties of such particles to escalate the dose to the tumor while simultaneously limiting the dose to nearby structures, thereby enhancing the therapeutic ratio and potentially improving cancer cure rates. This review summarizes the rationale for and challenges associated with the use of charged particles for lung cancer therapy and reviews the clinical experience to date with using protons and carbon ions for early-stage and locally advanced stage non-small cell lung cancer.
Lung cancer is the most common type of cancer worldwide, accounting for over 1.35 million cases per year [Citation1]. Although surgery is the standard treatment for early-stage cancers, radiotherapy is a good option for patients with medically inoperable disease, and for patients with locally advanced disease, radiotherapy given with chemotherapy is considered the standard of care. Recent advances in photon-based radiotherapy involving three dimensional conformal radiotherapy (3D-CRT) and intensity-modulated radiotherapy (IMRT) allow the escalation of doses beyond the traditional 60-Gy limit established by the Radiation Therapy Oncology Group (RTOG) 7301 trial. Most of the trials that established the importance of sequential chemotherapy, and currently concurrent chemoradiotherapy, used radiation doses of 60 Gy, which is now considered insufficient for tumor eradication. Unfortunately, for most patients with locally advanced disease, the median survival time is only 16–24 months, with local recurrence accounting for 40–50% of failures. To date, radiation doses above 60 Gy have been tested only in phase I or II studies, with the currently enrolling RTOG 0617 trial testing whether 74 Gy is superior to the traditional standard of 60 Gy.
Although tumor control is clearly related to radiation dose, higher doses come at the cost of toxicity to normal tissues. Such toxicity is often the limiting factor in dose-escalation trials. Incremental advances in radiation technologies can help minimize the radiation dose to normal structures and reduce acute and late normal tissue toxicities. Vital organs particularly relevant in the treatment of thoracic malignancies are the esophagus, lung, and heart; other important structures or tissues include the brachial plexus, skin, and chest wall. Charged particle therapy, mostly with protons or carbon ions, has the dosimetric advantage over photon-based radiation in its ability to limit the dose to these normal structures, which presumably would enhance the therapeutic ratio and the potential cancer cure rates via dose escalation. Despite the high capital costs associated with charged particle therapy, the increasing demand for such therapy in cancer treatment, particularly proton therapy, is evidenced by the numbers of facilities built or being built worldwide. At present, 29 proton centers and six carbon centers are open worldwide, with nine proton centers operational in the US alone, and many more are being planned. More than 78 000 patients worldwide have been treated so far with charged particle therapy.
In this review, we summarize the rationale for and challenges in the use of charged particles for lung cancer therapy and review the updated clinical experience with using protons and carbon ions for early-stage and locally advanced non-small cell lung cancer (NSCLC).
Depth-dose distribution and radiobiology of charged particle therapy
Charged particle therapy involves the use of ionizing radiation, such as protons or carbon, to treat cancer. Charged particles have very different physical properties than do x-rays. X-rays enter the body, deposit the greatest amount of energy at some depth below the skin surface (called Dmax), and leave a diminishing trail of attenuated energy as they exit the body. Although the convergence of several beams from various angles allows dose build-up in the tumor, the entrance and exit of each beam creates a “dose bath” within the patient, the amount of which relates to the number of beams and the fractional weighted dose of each beam.
Interest in the use of charged particles for cancer treatment comes from the fundamental difference in how charged particles interact with tissue. When a “fast” charged particle moves through matter, it interacts with the electrons within atoms and causes ionization. This ionization deposits energy and dose along its path. The cross-section of the probability of this interaction increases as the particle slows down in tissue, with the energy loss per unit path length being relatively constant until it reaches a “peak” in energy deposition that occurs at a depth that is a function of the energy and nature of the charged particle. This peak is known as the Bragg peak, named after William Henry Bragg who described it in 1903. Beyond the Bragg peak, practically no dose exits the body. This phenomenon can be exploited since it effectively concentrates the dose deposited by the ion beams in the tumor while minimizing the effect on the surrounding healthy tissue. The Bragg peak itself is generally too narrow to be clinically useful; to cover the entire volume of a tumor at depth requires the beam to be modulated by superpositioning several Bragg peaks of decreasing energies to create the so-called spread-out Bragg peak (SOBP) that provides a uniform dose that covers the target. Although the entrance dose of the SOBP is substantially greater than that of a single Bragg peak, it is still significantly lower than the entrance dose from photon radiation. The dose immediately beyond the Bragg peak is practically zero, with very little neutrons contributing to the dose beyond the peak.
Not only does charged particles have a large dosimetric advantage but it could also have a biological advantage in terms of how various charged particles interact with matter. The biological interaction is related to the amount of energy transferred to matter over a specified path length (otherwise known as linear energy transfer [LET]). For particles such as protons and helium, the LET is nearly equivalent to that of photons, and therefore the relative biological effectiveness (RBE) is also nearly equivalent (the RBE for protons:photons equals 1.1). For heavier charged particles such as carbon, the density of ionization is greater at the end of their range, which causes greater damage to the DNA within a cell. This results in carbon ions having a higher RBE of 1.5–3.
Dosimetric advantages of charged-particle therapy in lung cancer
Numerous planning studies have been done to demonstrate the utility and dosimetric superiority of charged particle therapy in comparison with photon-based radiotherapy, although most of these comparative studies involved comparing proton beam therapy with photon therapy. In one of the earliest such studies, Lee et al. compared the maximum tolerated dose that could be achieved for 3D-CRT and passive-scattering proton therapy by using some pre-set limits to the spinal cord (maximum dose <48 Gy) and lung (the volume exposed to 25 Gy or more [total lung V25] <40%, and the V25 of the contralateral lung <20%) [Citation2]. Of the 13 patients with stage III NSCLC included in this planning comparison study, two could not be treated with either 3D-CRT or proton therapy, even to a dose of 60 Gy, without exceeding these pre-set limits. However, the dose that could be delivered to nine of the 13 patients could be escalated to 90 Gy with proton therapy, but 3D-CRT could achieve this dose for only four of the 13 patients. Chang et al. [Citation3] conducted a planning study based on the treatment of 10 patients with medically inoperable stage I NSCLC and 15 patients with stage III NSCLC with conventionally fractionated photon radiotherapy, given as either 3D-CRT to 66 Gy or IMRT to 63 Gy. Plans for those patients were then generated for proton therapy, escalated to 87.5 Gy (RBE) (the unit referring to the modification of the physical dose of a charged particle by its relative biological effectiveness) in 35 fractions for stage I tumors or 74 Gy (RBE) for stage III tumors. For stage I cases, in comparison with 66 Gy given as 3D-CRT, the proton plans for delivering 87.5 Gy (RBE) in 35 fractions had better V5 (13.4% vs. 31.8%), V10 (12.3% vs. 31.8%), and V20 (10.9% vs. 15.8%) (p = 0.002). For stage III disease, proton plans for delivering 74 Gy (RBE) also had significantly better total lung V5 (39.7% vs. 54.1%), V10 (36.6% vs. 46.9%), and V20 (31.6% vs. 34.8%) (p = 0.002) than did 66 Gy given as 3D-CRT. In comparison to the IMRT plans, proton plans could still spare more lung, spinal cord, and heart, regardless of whether the plan used the 63-Gy dose or the 74-Gy dose. MacDonald et al. conducted a similar theoretical comparison study to analyze the dosimetric differences between passive-scattering protons, active scanning protons, and stereotactic body (photon) radiotherapy (SBRT) for the treatment of early-stage NSCLC [Citation4]. In comparison with SBRT to a dose of 60 Gy in 3 fractions, the proton plans produced better (lower) mean lung dose, lung integral doses, and dose to the esophagus, bronchial tree, and spinal cord, with a slight trade-off of higher maximum doses to skin and ribs but lower median dose to these structures than SBRT. Similarly, Register et al. compared passive scattering protons or intensity-modulated protons with SBRT to 50 Gy in 4 fractions [Citation5]. They found that both ways of delivering protons led to significantly lower mean lung doses (3.5–5.4 Gy) and lower doses to the aorta, brachial plexus, heart, large vessels, and spinal cord than SBRT. Hoppe et al. analyzed plans from eight patients treated with (photon) SBRT to 48 Gy in 4 fractions [Citation6] in comparison with new plans based on 3D conformal double-scatter proton therapy. Relative to SBRT, the proton plans showed significant dosimetric improvements to the organs at risk, particularly the lung (mean lung dose 2.17 Gy, p = 0.01), and exposed smaller volumes of the lung to various doses (V5, V10, V20, and V40). Similar findings in planning studies of patients with stage I NSCLC have also been reported by other investigators [Citation7,Citation8].
The dosimetric advantages of passive-scattering proton therapy, although obvious for stage I NSCLC where the disease is confined to the ipsilateral lung parenchyma, are more limited for patients with locally advanced disease, particularly if the disease is more centralized and encroaches into the contralateral lung space. In these situations, IMRT, despite a greater low-dose spread throughout both lungs, may yield better plans because of the greater conformality. However, with the advent of active-scanning proton therapy or intensity-modulated proton therapy, protons could again have the advantage. The benefit of using intensity-modulated proton therapy rather than photon-based IMRT for the treatment of stage IIIB NSCLC was reported in a virtual study by Zhang et al. [Citation9]. In that study, 10 patients with stage IIIB NSCLC underwent treatment planning for IMRT to 60–63 Gy, for passive-scattering proton therapy to 74 Gy (RBE), and for intensity-modulated proton therapy to all dose levels. These patients were selected because IMRT plans for them could not be designed that would meet normal tissue constraints (mean lung dose <20 Gy, V20 <35%) when doses were greater than 63 Gy. Compared with IMRT, intensity-modulated proton therapy was able to spare more lung, heart, spinal cord, and esophagus, even with dose escalation to 83.5 Gy, with a mean maximum tolerated dose of 74 Gy. Compared with passive-scattering proton therapy, intensity-modulated proton therapy allowed further dose escalation from 74 Gy to a mean maximum tolerated dose of 84.4 Gy (range 79.4–88.4 Gy). Use of intensity-modulated proton therapy allowed all normal-tissue tolerance measures to be kept below or at the same levels and low-dose contralateral lung spread was better than IMRT; intensity-modulated proton therapy also provided better target volume coverage than did passive-scattering proton therapy.
Challenges with the use of charged particle therapy
Thus in theory, proton therapy is clearly superior to photon-based radiation in terms of improving the therapeutic ratio because of its ability to limit normal tissue exposure while simultaneously achieving better tumor control because of the potential for safe dose escalation. However, the physical properties of finite range in tissue that makes proton therapy so appealing are also potentially problematic, especially in lung cancer, where tumor motion due to respiration is a key concern. Uncertainties such as these make proton planning a major challenge. Extreme care must be taken to consider the need to compensate for tumor motion and changes in lung density due to respiration. Moyers et al. performed a planning study in which three sets of plans were generated for a single patient to determine which plan provided the best target coverage; each plan involved different apertures and different definitions of distal margins [Citation10]. These investigators proposed that uncertainties in proton range with regard to respiration-induced tumor motion and lung density changes should be assessed separately for each beam direction, so that some amount of dosimetric uncertainty is built in into the planning of each beam. Engelsman et al. studied the effect of setup uncertainties and respiratory motion on cumulative doses to the lung tumor in another planning study [Citation11]. Adding various amount of “smearing distances” (by modifying the range compensator to ensure target coverage in the presence of volume uncertainties) applied to combinations of potential errors due to breathing motion and setup inconsistencies allowed better coverage of the clinical target volume (CTV) but simultaneously increased the dose to normal tissue distal to the CTV. Accounting for motion is a key step in the planning process, and knowing which datasets to use for planning is very important. Kang et al. compared plans from 10 patients using four different four dimensional computed tomography (4D-CT) imaging datasets: (1) the average CT; (2) the free-breathing CT; (3) the maximum intensity projection CT; and (4) the average CT in which the voxels within the internal gross tumor volume GTV (iGTV; designed to account for motion) were replaced with a constant density (AVE_RIGTV) [Citation12]. Using a 1-cm smearing parameter, the AVE_RIGTV plans achieved the best overall target coverage and critical structure sparing, the maximum intensity projection plan resulted in unnecessarily large treatment volumes and normal tissue dosing, and both the average and the free-breathing plans had inadequate 4D target coverage.
Although the motion and lung density uncertainties can be accounted for during the planning process by adding generous internal and smearing margins, practical issues of inconsistencies in patient setup and positioning and changes in tumor volume resulting from the treatment must also be accounted for. These issues were analyzed in a study by Hui et al. [Citation13] in which weekly 4D-CT scans were acquired for eight patients during seven weeks of IMRT. Passive-scattering proton therapy plans were designed for each patient, and the dose distributions were recalculated at end-inspiration and end-expiration phases of each of the weekly 4D-CT scans. This group observed that target CTV coverage showed little change over the course of the treatment if tumor motion had been accounted for during the original proton therapy planning; the interpretation was that imaging during the original 4D-CT simulation can predict the pattern of tumor motion over the course of therapy. However, up to 25% of the CTV could be missed over the course of therapy if the setup relied on skin markers only, compared with an up to 9% error if daily bone registration was used, indicating that daily x-ray images and bony markers should be used to align the patient before each proton therapy session. Although normal tissue doses were nearly the same for IMRT and the two sets of proton plans, all eight patients had a mean 4% increase in V5 to the contralateral lung and a 4.4-Gy increase in spinal cord dose over the course of the seven-week treatment. Moreover, patients with the largest CTV changes over time had the largest differences in contralateral lung dose as well as losing CTV coverage as a result.
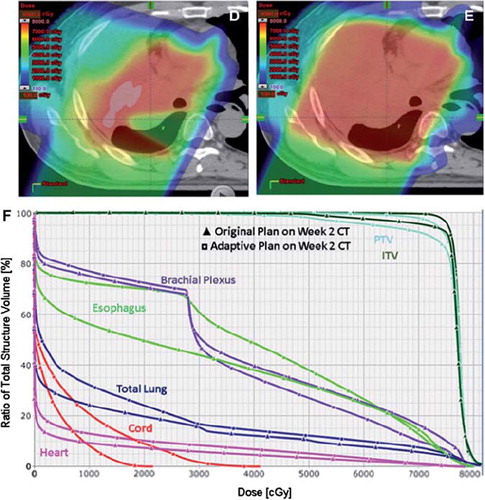
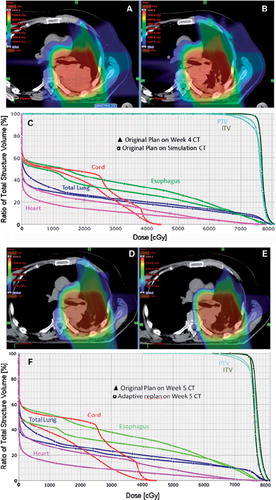
shows images of a patient with stage III NSCLC who has enrolled on the current clinical trial comparing IMRT and protons. During the second week of a routine weekly verification resimulation CT, there was evidence of atelectasis in the right upper lobe which created a cold spot in the GTV. DVH analysis of the initial plan compared to the dosimetric alteration due to the atelectasis. Except for the brachial plexus, there was an overall increase in mean and absolute doses to all the indicated normal structures (esophagus, heart, spinal cord) and a slight under dosing of the ITV and PTV. shows images of a proton beam dose distribution from the original plan in a patient with T3N2 adenocarcinoma. During week 4 of routine repeat CT simulation, there was clear evidence of response to therapy with necrotic hollowing of the tumor center. This alters the proton beam dose distribution, with increased dose to adjacent normal structures but without a compromise to tumor coverage. These findings illustrate the sensitivity of the proton dose distribution to uncertainties in setup accuracy and tumor changes. They highlight the critical need for weekly CT imaging to assess tumor changes during treatment, with adaptive re-planning implemented as needed.
Figure 1. Proton Beam dosimetry is sensitive to lung density changes. A) Axial CT imaging of the proton beam dose distribution of the original plan for the treatment of a patient with stage III NSCLC. During the second week of a routine weekly verification resimulation CT, there was evidence of atelectasis in the right upper lobe (B). C) DVH analysis of the initial plan compared to the dosimetric alteration due to the atelectasis. Except for the brachial plexus, there was an overall increase in mean and absolute doses to all the indicated normal structures (esophagus, heart, spinal cord) and a slight under dosing of the ITV and PTV. Compared to D, panel E is an adaptive plan that was done in order to improve tumor coverage. However in order to achieve this, there had to be a compromise in the dose to normal tissues with a slightly increased doses compared to the initial plan. This is reflective in the DVH in panel F.
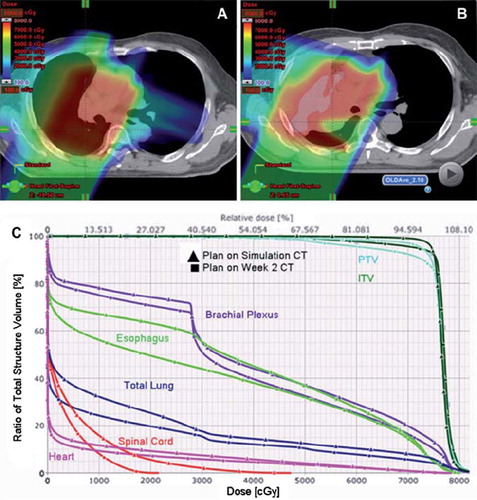
Figure 2. Proton Beam dosimetry is sensitive to tumor response to treatment. A) Axial CT imaging of a proton beam dose distribution from the original plan in a patient with T3N2 adenocarcinoma. B-C) During week 4 of routine repeat CT simulation, there was clear evidence of response to therapy with necrotic hollowing of the tumor center. This alters the proton beam dose distribution, with increased dose to adjacent normal structures but without a compromise to tumor coverage. D-E) Adaptive plan was done on the week 5 CT simulation scan in order to improve the dose distribution, with DVH in F) showing reduced normal tissue doses compared to what would have been delivered if no adaptive planning was performed.
Current status of proton beam therapy for lung cancer
Despite the technical challenges associated with using particle therapy for lung cancer, where tissue heterogeneity causes significant uncertainty in dose, several trials of proton therapy have been conducted in patients with NSCLC. However, these trials have involved mostly patients with early-stage disease and dose-escalated or accelerated proton therapy. Clinical evidence is seriously lacking on the use of particle therapy for locally advanced NSCLC, or the use of proton therapy in combination with chemotherapy. The current experience with proton therapy for early-stage and locally advanced NSCLC is summarized in the following sections.
Early-stage non-small cell lung cancer
Stage I NSCLC is curable in a relatively high proportion of patients if the disease can be resected, but many patients cannot undergo surgery because of underlying cardiac and pulmonary diseases, and radiation therapy with curative intent is often successful. The radiation treatment of choice for early-stage NSCLC has evolved from conventional fractionated radiotherapy to the currently more popular image-guided SBRT or hypofractionated radiotherapy. Interest in charged particle therapy for lung cancer has increased substantially as well. Several studies of hypofractionated particle therapy for early-stage NSCLC have been reported. Extensive systematic reviews of the literature in search of clinical evidence to support particle therapy for lung cancer [Citation14–18] have led to a general impression that, because of the limited evidence accrued to date, the advantage of particle therapy over image-guided SBRT or hypofractionated photon therapy for early-stage lung cancer has yet to be established, and well-designed randomized clinical trials are needed.
Image-guided hypofractionated SBRT has produced excellent local control rates, ranging from 80% to 97.6%, and survival rates of about 55% with minimal toxicity for peripherally located stage IA NSCLC [Citation19–22]. A biologically effective dose (BED) of 100 Gy10 or higher is associated with better local control rates (91.9% vs. 73.6%) and survival rates (88.4% vs. 69.4%) than is a BED of less than 100 Gy10 [Citation23].
Although peripheral lesions can be treated to high BEDs, high-BED treatments for centrally located lesions can result in considerable long-term toxicity because of the proximity of critical structures such as bronchi, major vessels, heart, spinal cord, esophagus, and trachea [Citation24]. With image-guided interventions and other improvements in the technology, proton therapy may allow the dose to be escalated or the treatment to be accelerated, which presumably would led to better local control and reduced toxicity, particularly for patients with centrally located or bulky early-stage NSCLC who are not good candidates for SBRT.
An early prospective study from Loma Linda [Citation25] enrolled 37 patients with stage I-IIIA, medically inoperable NSCLC. Eighteen patients with good pulmonary and cardiac function received 45 Gy photon radiation to the mediastinum and gross tumor volume, with a concurrent proton boost to the gross tumor volume of an additional 28.8 Gy (RBE), for a total of 73.8 Gy (RBE) given over five weeks. The other 19 patients, who had poor cardiopulmonary function, received proton therapy to the gross tumor volume only, with 51 Gy (RBE) given in 10 fractions over a two-week period. Only two patients developed symptomatic pneumonitis. The actuarial disease-free survival rate at two years for the entire group was 63% (86% for patients with stage I disease). Local disease was controlled in 87% of patients. A subsequent study from the same institution [Citation26] included 68 patients with clinical stage I disease treated with 50 Gy (RBE) in 10 fractions over two weeks or with 60 Gy (RBE) in 10 fractions over two weeks. No patients developed symptomatic pneumonitis or late esophageal or cardiac toxicity. At three years, the local control was 74% and the disease-specific survival rate was 72%. Local control rates were better for T1 tumors (87%) than for T2 tumors (49%), and survival rates may have been better as well. Predicted survival rates calculated with the Charlson Comorbidity Index method were 67% at two years and 50% at four years, and the actual comorbidity-specific survival rates were 64% at two years and 45% at four years [Citation27].
Shioyama et al. [Citation28] described 51 patients with NSCLC who were treated with proton therapy, to a median dose of 76 Gy (RBE) (median fraction size, 3.0 Gy (RBE)). The five-year overall survival rates were 70% for nine patients with stage IA disease and 16% for 19 patients with stage IB disease (p < 0.05). The five-year in-field local control rate was higher for patients with stage IA disease (89%) than for those with stage IB disease (39%). Forty-seven patients (92%) experienced acute lung toxicity of grade 1 or less, three had grade 2, one had grade 3, and none experienced grade 4 or higher toxicity.
Nihei et al. [Citation29] reported the results from their preliminary study of 37 patients with stage I NSCLC who received 70 Gy to 94 Gy (RBE) delivered in 20 fractions. At two years, the progression-free survival rate was 80% and the overall survival rate was 84%; local-regional relapse-free survival rates were 79% for patients with stage IA disease and 60% for those with stage IB disease. No serious acute toxicity was observed, and only three patients developed grade 2 or 3 chronic lung toxicity.
In a phase I/II trial of proton therapy for NSCLC, Chang et al. [Citation30] analyzed the toxicity and patterns of failure after ablative doses of proton therapy for 18 patients with medically inoperable T1N0M0 (centrally located) or T2-3N0M0 (at any location) disease. The dose was 87.5 Gy (RBE) given at 2.5 Gy (RBE) per day. All patients underwent treatment simulation with 4D-CT to define the internal gross tumor volumes on maximum intensity projection images, and all patients underwent repeat 4D-CT simulations during treatment to assess the need for plan modification. At a median follow-up time of 16.3 months (range, 4.8–36.3 months), no patient had experienced grade 4 or 5 toxicity. The most common adverse effect was dermatitis (grade 2, 67%; grade 3, 17%), followed by grade 2 fatigue (44%), grade 2 pneumonitis (11%), grade 2 esophagitis (6%), and grade 2 chest wall pain (6%). The local control rate was 88.9%, regional lymph node failure rate 11.1%, and distant metastasis rate 27.8%. Twelve patients (67%) were still alive at the last follow-up; five had died of metastatic disease and one of preexisting cardiac disease. These results suggest that ablative dose proton therapy is safe and can provide a good treatment option for patients with centrally located early-stage lung cancer [Citation30].
Iwata et al. [Citation31] reported the results of high-dose proton or carbon-ion therapy for 80 patients with stage I NSCLC. In that study, 57 patients were treated with proton therapy and 23 patients with carbon-ion therapy in one of three treatment protocols. The first protocol involved an 80 Gy (RBE) dose of protons given in 20 fractions, the second 60 Gy (RBE) protons in 10 fractions, and the third carbon ions to 52.8 Gy (RBE) given in 4 fractions. Both proton and carbon-ion therapy plans were made for each patient, and the plan that best met normal tissue dose constraints was used. At a median follow-up time of 35.5 months for living patients, the three-year overall survival, cause-specific survival, and local control rates were as follows: 75% (IA, 74%; IB, 76%), 86% (IA, 84%; IB, 88%), and 82% (IA, 87%; IB, 77%), respectively. No differences were found among the protocols in terms of treatment results, and only one patient experienced grade 3 pulmonary toxicity [Citation31].
Recently, Peeters et al. [Citation32] compared the capital and operational costs, cost per fraction, and treatment costs for three types of charged-particle therapy facilities (combined carbon-ion/proton, proton-only, and photon). The results showed that the cost ratio for particle/photon therapy was 4.8 for the combined proton/carbon and 3.2 for the proton-only facility. In terms of the types of tumors treated, the cost differences between particle and photon therapies were relatively small for lung and prostate cancer and larger for skull-base chordomas and head and neck tumors, the investigators suggested that reducing the number of fractions of particle therapy might further reduce its costs [Citation32].
summarizes representative results on local tumor control and survival for patients with early-stage NSCLC after proton therapy, carbon-ion therapy, or SBRT [Citation19,Citation22,Citation24,Citation26,Citation29–31,Citation33–35]. These clinical studies have shown the safety and efficacy of proton therapy for early-stage NSCLC compared with photon SBRT. However, the hypothesized higher therapeutic ratio of particle therapy compared with SBRT photon therapy for early-stage NSCLC remains to be established. A prospective randomized trial comparing proton therapy and SBRT for early-stage NSCLC is being developed at The University of Texas MD Anderson Cancer Center.
Table I. Results of high-dose proton therapy, carbon-ion therapy, and stereotactic body radiotherapy for stage I non-small cell lung cancer.
Locally advanced non-small cell lung cancer
The therapeutic ratio of particle therapy versus photon radiation therapy for locally advanced (stage IIB-III) NSCLC should be much higher than that for early-stage NSCLC. Locally advanced NSCLC is still a deadly disease and poses a significant treatment challenge. Radiotherapy is the mainstay of treatment for disease at this stage, and the current standard of care includes chemotherapy as well as radiation. Although locally advanced NSCLC is quite likely to metastasize, many patients die of the consequences of uncontrolled intrathoracic tumor, and hence therapies that improve local control would be valuable in terms of survival. Use of induction chemotherapy before radiotherapy improves survival over radiotherapy alone [Citation36]. However, in a French trial, induction chemotherapy followed by radiation therapy with doses of 65 Gy in 26 fractions in 6.5 weeks controlled the primary tumor in only 20% of patients [Citation37]. Giving cisplatin-based chemotherapy daily or weekly during radiotherapy has produced higher local-regional control rates and survival rates [Citation38]. Other types of chemotherapy given with radiotherapy have also led to improved local control and survival compared with giving the chemotherapy first [Citation39]. Local tumor recurrence clearly contributes to both morbidity and mortality in locally advanced NSCLC.
An early attempt to intensify radiotherapy with higher total doses and twice-daily sessions, given with concurrent chemotherapy produced substantial toxicity, with no improvement in survival rates [Citation40]. Advances in technology since the time of that study have since made dose intensification possible. For example, use of 3D-CRT produced less esophageal toxicity than did use of two dimensional (2D) techniques among 215 patients treated at MD Anderson Cancer Center [Citation41]. The further increases in conformality and normal-tissue sparing with IMRT relative to 3D-CRT led to a median absolute reduction of 7% in V10 and 10% in V20; a decrease of >2 Gy in the total mean lung dose; and predicted reductions in the risk of treatment-related pneumonitis from 13% to 36% with the 3D-CRT plans to 7%–9% with the IMRT plans [Citation42]. This prediction was confirmed in a retrospective study of IMRT with concurrent chemotherapy for the definitive treatment of advanced NSCLC, in that the rates of grade >3 pneumonitis were 9% for IMRT versus 33% for 3D-CRT (p = 0.028) [Citation43], and IMRT was associated with better overall survival as well [Citation44]. These reductions in toxicity were directly related to significant reductions in the irradiated volumes of and doses to normal tissue (mean doses to normal lung were 16.6 Gy [range, 2.5–28.5] for IMRT and 22.1 Gy [range, 1.0–38.9] for 3D-CRT). IMRT also led to significant decreases in median irradiated volumes at several dose levels, including V15–V65 (p < 0.0001). However, V5, V10, and V70 were no different between the two techniques, suggesting that IMRT is of limited benefit in terms of minimizing lung exposure to low-dose radiation [Citation43]. The dosimetric characteristics of protons (the Bragg peak and finite range in material that deposits a sharply increased dose at the target and none beyond it) make proton therapy an attractive alternative to IMRT, as it provides the potential for further radiation dose intensification without an increase in toxicity.
A recently reported retrospective study [Citation45] compared the toxicity of proton therapy for 62 patients with NSCLC (treatment period 2006–2008) with toxicity for patients with similar disease given 3D-CRT (n = 74; treatment period 2001–2003) or IMRT (n = 66; treatment period 2003–2005). All patients received platinum- and taxane-based chemotherapy concurrently with the radiotherapy. Median follow-up times were 15.2 months for the proton group, 17.9 months for the 3D-CRT group, and 17.4 months for the IMRT group. The median total radiation dose was 74 Gy (RBE) for the proton group versus 63 Gy for both of the other groups. Rates of severe (grade ≥3) pneumonitis and esophagitis in the proton group (2% and 5%) were lower despite the higher radiation dose (3D-CRT, 30% pneumonitis and 18% esophagitis; IMRT, 9% pneumonitis and 44% esophagitis; p < 0.001 for all) [Citation45]. Furthermore, proton therapy produced less hematologic toxicity than 3D-CRT or IMRT in the same patient populations [Citation46]. These clinical results suggest that protons may “open a therapeutic window” in combined-modality therapy by reducing normal tissue toxicity, thereby allowing higher total doses to the tumors.
Another retrospective study of protons as single-modality therapy for locally advanced lung cancer showed quite favorable local control and survival rates in 65 patients with poor performance status [Citation47]. Patients in that study were those with stage II or III NSCLC who could not or chose not to undergo surgery or chemotherapy. The median proton dose given was 78.3 Gy (RBE) (range, 67.1–91.3 Gy (RBE)). At a median follow-up time of 16.9 months, local progression-free survival rates for all patients were 93.3% at one year and 65.9% at two years. Four patients (11%) developed local recurrence, 13 (37%) developed regional recurrence, and seven (20%) developed distant metastases. The progression-free survival rates were 59.6% at one year and 29.2% at two years, and the overall survival rates were 81.8% at one year and 58.9% at two years. No grade 3 or higher toxicity was observed [Citation47].
Early results on toxicity, failure patterns, and survival in an ongoing phase II study of high-dose proton therapy and concurrent chemotherapy for patients with locally advanced NSCLC and good performance status (clinicaltrials.gov identifier NCT00495170) were recently reported [Citation48]. Forty-four patients with stage III NSCLC (staged with positron emission tomography (PET)/CT scanning) were treated with 74 Gy (RBE) of passive-scattering protons with weekly carboplatin (2 area under the curve units) and paclitaxel (50 mg/m2). All treatments were simulated with 4D-CT to account for tumor motion, and treatment simulation was repeated during the treatment period to determine the need for adaptive replanning. The median follow-up time was 19.7 months (range, 6.1–44.4 months), and the median overall survival time was 29.4 months. No patient experienced grade 4 or 5 proton-related adverse events. The most common non-hematologic grade 3 toxicities were dermatitis (n = 5), esophagitis (n = 5), and pneumonitis (n = 1). Nine patients (20.5%) experienced local disease recurrence, but only four (9%) had isolated local failure. Four patients (9%) had regional lymph node recurrence, but only one (2.3%) had isolated regional recurrence. Nineteen (43.2%) patients developed distant metastasis. The overall survival and progression-free survival rates at one year were 86% and 63%. These encouraging, albeit preliminary, results suggest that proton therapy may have a higher therapeutic ratio than IMRT when used with concurrent chemotherapy for locally advanced NSCLC.
We are currently conducting a Bayesian randomized trial to compare image-guided adaptive conformal photon therapy with image-guided proton therapy, both with concurrent chemotherapy, for locally advanced NSCLC in terms of time to grade ≥ 3 radiation pneumonitis (defined according to the Common Terminology Criteria for Adverse Events, version 3.0) (clinicaltrials.gov identifier NCT00915005). This trial was designed to test the validity of the assumption that passive-scattering proton therapy for locally advanced NSCLC will lead to greater sparing of critical normal tissues than will IMRT, and to assess the probability that the prescribed dose of 74 Gy (or Gy (RBE)) can be achieved by either method without exceeding normal tissue constraints. In a preliminary assessment [Citation49], treatment plans were designed for the first 18 patients with stage III NSCLC enrolled in this randomized trial. Planning objectives included covering ≥99% of the planning target volume with ≥95% of the prescribed dose while maintaining the following normal-tissue dose limits: normal lung V20 ≤37%; mean lung dose ≤22 Gy (RBE); 1/3 of the esophagus receiving ≤65 Gy (RBE), 2/3, ≤55 Gy (RBE), and 100%, ≤45 Gy (RBE); 1/3 of the heart receiving ≤60 Gy (RBE), 2/3, ≤45 Gy (RBE), and 100% ≤30 Gy (RBE); maximum dose to the spinal cord ≤50 Gy (RBE); and maximum dose to the brachial plexus ≤60 Gy (RBE). If photon plans could be designed that met these criteria then the patients were to be randomized between proton therapy and IMRT; otherwise, the patients would be treated with protons (with the assumption being that protons will always be better for achieving normal tissue dose constraints). Relevant dose and dose–volume indices of pairs of plans were to be compared, and the same indices were compared for plans that had been renormalized such that the dose to 95% of the planning target volume was the highest of the two modalities. Interestingly, for un-renormalized dose distributions, the average lung V20, the most important planning constraint, was lower for IMRT than for passive-scattering proton therapy (29.3% vs. 32.1%, p = 0.01). Similarly, V30 and V40 were also lower for IMRT than for proton therapy. On the other hand, lung V5 and mean heart dose were significantly lower for proton therapy (V5 42% for protons vs. 53.9% for IMRT, p = 0000; mean heart dose 7.4 Gy (RBE) for protons vs. 12.2 Gy (RBE) for IMRT, p = 0.005). Apparent differences in mean lung dose (which seemed slightly higher for proton therapy than for IMRT) and other indices were not statistically significant. The same pattern was seen for dose distributions that had been normalized to the same planning-target-volume dose. For 10 of the 18 cases, both IMRT and proton plans could achieve the intended target dose level. Higher target doses could be delivered with IMRT in 11 cases, with proton therapy in six cases, and the same in one case. Possible explanations for these findings may be the relative immaturity of proton therapy technology and processes, the ability of IMRT to skirt normal critical structures, and the enlargement of the irradiated volume to accommodate the greater sensitivity of protons to inter- and intra-fractional uncertainties. Nevertheless, despite the apparent dosimetric advantage of IMRT in this study, early results from prior non-randomized trials of passive-scattering proton therapy for NSCLC to a dose of 74 Gy (RBE) indicate lower lung and esophageal toxicity despite the escalated tumor dose [Citation45,Citation48]. This paradox suggests possible equipoise between the two treatment arms and that factors other than, or in addition to, V20 and mean lung dose may be responsible for toxicities, such as the lower heart dose and reduced dose bath from proton therapy [Citation49]. The findings of this study will highlight the importance of prospective randomized trials to establish level 1 evidence for particle therapy for lung cancer.
Summary
Particle therapy for lung cancer makes sense in theory; planning studies have shown it to be less toxic than photon therapy, and it has shown encouraging early results in small studies from Japan and the US. Given the advancements in image-guided SBRT for early-stage NSCLC, any advantages of particle therapy over photon therapy for this purpose remain to be determined. For locally advanced NSCLC, retrospective and single-arm prospective studies suggest that proton therapy has the better toxicity profile. An ongoing prospective randomized trial is testing the hypothesis that proton therapy spares normal tissue better than IMRT and will be less toxic in combination with concurrent chemoradiation therapy.
Acknowledgements
The authors acknowledge Jaques Bluett, CMD, for contributing the images for and and Ms. Christine F. Wogan for her editorial assistance.
Disclosure of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- International Agency for Research on Cancer W. lung cancer incidence and mortality worldwide in 2008. Globocan 2008 Cancer Fact Sheet, 2008.
- Lee CHM, Tait D, Nahum AE, Webb S. Comparison of proton therapy and conformal X-ray therapy in non-small cell lung cancer (NSCLC). Br J Radiol 1999;72:1078–84.
- Chang JY, Zhang X, Wang X, Kang Y, Riley B, Bilton S, . Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:1087–96.
- Macdonald OK, Kruse JJ, Miller JM, Garces YI, Brown PD, Miller RC, . Proton beam radiotherapy versus three-dimensional conformal stereotactic body radiotherapy in primary peripheral, early-stage non-small-cell lung carcinoma: A comparative dosimetric analysis. Int J Radiat Oncol Biol Phys 2009;75:950–8.
- Register SP, Zhang X, Mohan R, Chang JY. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2010 Epub Jul 7.
- Hoppe BS, Huh S, Flampouri S, Nichols RC, Oliver KR, Morris CG, . Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: A dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol 2010;97:425–30.
- Wang C, Nakayama H, Sugahara S, Sakae T, Tokuuye K. Comparisons of dose-volume histograms for proton-beam versus 3-D conformal X-ray therapy in patients with stage I non-small cell lung cancer. Strahlenther Onkol 2009;185:231–4.
- Auberger T, Seydl K, Futschek T, Sztankay A, Sweeney RA, Lukas P. Photons or protons: Precision radiotherapy of lung cancer. Strahlenther Onkol 2007;183:3–6.
- Zhang X, Li Y, Pan X, Xiaoqiang L, Mohan R, Komaki R, . Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: A virtual clinical study. Int J Radiat Oncol Biol Phys 2010;77:357–66.
- Moyers MF, Miller DW, Bush DA, Slater JD. Methodologies and tools for proton beam design for lung tumors. Int J Radiat Oncol Biol Phys. 2001;49:1429–38.
- Engelsman M, Kooy HM. Target volume dose considerations in proton beam treatment planning for lung tumors. Med Phys 2005;32:3549–57.
- Kang Y, Zhang X, Chang JY, Wang H, Wei X, Liao Z, . 4D Proton treatment planning strategy for mobile lung tumors. Int J Radiat Oncol Biol Phys 2007;67:906–14.
- Hui Z, Zhang X, Starkschall G, Li Y, Mohan R, Komaki R, . Effects of interfractional motion and anatomic changes on proton therapy dose distribution in lung cancer. Int J Radiat Oncol Biol Phys 2008;72:1385–95.
- Bjelkengren G, Glimelius B. The potential of proton beam radiation therapy in lung cancer (including mesothelioma). Acta Oncol 2005;44:881–3.
- Pijls-Johannesma M, Grutters JPC, Lambin P, Ruysscher DD. Particle therapy in lung cancer: Where do we stand? Cancer Treat Rev 2008;34:259–67.
- Pijls-Johannesma M, Grutters JPC, Verhaegen F, Lambin P, De Ruysscher D. Do we have enough evidence to implement particle therapy as standard treatment in lung cancer? A systematic literature review. Oncologist 2010;15:93–103.
- Grutters JPC, Kessels AGH, Pijls-Johannesma M, De Ruysscher D, Joore MA, Lambin P. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: A meta-analysis. Radiother Oncol 2010;95:32–40.
- Grutters JP, Pijls-Johannesma M, Ruysscher DD, Peeters A, Reimoser S, Severens JL, . The cost-effectiveness of particle therapy in non-small cell lung cancer: Exploring decision uncertainty and areas for future research. Cancer Treat Rev 2010;36:468–76.
- Onishi H, Shirato H, Nagata Y, Hiraoka M, Fujino M, Gomi K, . Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94–100.
- Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, . Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427–31.
- Chang JY, Balter PA, Dong L, Yang Q, Liao Z, Jeter M, . Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;72:967–71.
- Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, . Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6.
- Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, . Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: Clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623–31.
- Timmerman R, McGarry R, Yiannoutsos C, Papiez L, Tudor K, DeLuca J, . Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833–9.
- Bush DA, Slater JD, Bonnet R, Cheek GA, Dunbar RD, Moyers M, . Proton-beam radiotherapy for early-stage lung cancer. Chest 1999;116:1313–9.
- Bush DA, Slater JD, Shin BB, Cheek G, Miller DW, Slater JM. Hypofractionated proton beam radiotherapy for stage I lung cancer. Chest 2004;126:1198–203.
- Bush DA, Do SY, Slater JD. Comorbidity-adjusted survival in early stage lung cancer patients treated with hypofractionated proton therapy. J Oncol 2010;2010:251208. Epub 2010 Dec 1.
- Shioyama Y, Tokuuye K, Okumura T, Kagei K, Sugahara S, Ohara K, . Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;56:7–13.
- Nihei K, Ogino T, Ishikura S, Nishimura H. High-dose proton beam therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:107–11.
- Chang JY, Komaki R, Wen HY, De Gracia B, Bluett JB, McAleer MF, . Toxicity and patterns of failure of adaptive/ablative proton therapy for early-stage, medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys Epub 2011 Jan 18.
- Iwata H, Murakami M, Demizu Y, Miyawaki D, Terashima K, Niwa Y, . High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer 2010; 116:2476–85.
- Peeters A, Grutters JPC, Pijls-Johannesma M, Reimoser S, De Ruysscher D, Severens JL, . How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother Oncol 2010; 95:45–53.
- Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, . Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290–6. Epub 2009 May 4.
- Miyamoto T, Baba M, Yamamoto N, Koto M, Sugawara T, Yashiro T, . for the Working Group for Lung Cancer. Curative treatment of stage I non-small-cell lung cancer with carbon ion beams using a hypofractionated regimen. Int J Radiat Oncol Biol Phys 2007;67:750–8.
- Miyamoto T, Baba M, Sugane T, Nakajima M, Yashiro T, Kagei K, . for Working Group for Lung Cancer. Carbon ion radiotherapy for stage I non-small cell lung cancer using a regimen of four fractions during 1 week. J Thorac Oncol 2007;2:916–26.
- Dillman RO, Seagren SL, Propert KJ, Guerra J, Eaton WL, Perry MC, . A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in Stage III non-small-cell lung cancer. N Engl J Med 1990; 323:940–5.
- Arriagada R, Le Chevalier T, Quoix E, Ruffie P, de Cremoux H, Douillard JY, . ASTRO (American Society for Therapeutic Radiology and Oncology) plenary: Effect of chemotherapy on locally advanced non-small cell lung carcinoma: A randomized study of 353 patients. GETCB (Groupe d’Etude et Traitement des Cancers Bronchiques), FNCLCC (Féderation Nationale des Centres de Lutte contre le Cancer) and the CEBI trialists.. Int J Radiat Oncol Biol Phys 1991; 20:1183–90.
- Schaake-Koning C, Maat B, Van Houtte P, van den Bogaert W, Dalesio O, Kirkpatrick A, . Radiotherapy combined with low-dose cis-diammine dichloroplatinum (II) (CDDP) in inoperable nonmetastatic non-small cell lung cancer (NSCLC): A randomized three arm phase II study of the EORTC Lung Cancer and Radiotherapy Cooperative Groups. Int J Radiat Oncol Biol Phys 1990;19:967–72.
- Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, . Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17: 2692–9.
- Curran WJ, Scott CB, Langer CJ, Komaki R, Lee J, Hauser S, . Long-term benefit is observed in a phase III comparison of sequential vs. concurrent chemo-radiation for patients with unresected stage III NSCLC: RTOG 9410 (abstract 2499). Proc Am Soc Clin Oncol 2003;22:621.
- Wei X, Liu HH, Tucker SL, Liao Z, Hu C, Mohan R, . Risk factors for acute esophagitis in non-small-cell lung cancer patients treated with concurrent chemotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:100–7.
- Murshed H, Liu HH, Liao Z, Barker JL, Wang X, Tucker SL, . Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;58: 1258–67.
- Yom SS, Liao Z, Liu HH, Tucker SL, Hu CS, Wei X, . Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2007;68: 94–102.
- Liao ZX, Komaki RR, Thames Jr HD, Liu HH, Tucker SL, Mohan R, . Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2010;76:775–81.
- Sejpal S, Komaki R, Tsao A, Chang JY, Liao Z, Wei X, . Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer Epub 2011 Jan 24. doi: 10.1002/cncr.25848.
- Komaki R, Sejpal SV, Wei X, Allen P, Chang JY, Liao Z, . Reduction of bone marrow suppression for patients with stage III NSCLC treated by proton and chemotherapy compared with IMRT and chemotherapy (abstract). Presented at Particle Therapy Cooperative Group (PTCOG) 47, May 19–24, 2008, Jacksonville, FL.
- Nakayama H, Satoh H, Sugahara S, Kurishima K, Tsuboi K, Sakurai H, . Proton beam therapy for stage II and III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys Epub 2010 Sep 30.
- Chang JY, Komaki R, Lu C, Wen HY, Allen PK, Tsao A, . Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer Epub 2011 Mar 22. doi: 10.1002/cncr.26080.
- Mohan R, Zhang X, Matney J, Bluett J, Dong L, Balter P, . IMRT vs. passively scattered proton therapy (PSPT) for locally advanced non-small cell lung cancer (LA NSCLC) randomized trial - is there equipoise? (abstract 1137). Int J Radiat Oncol Biol Phys 2010;78:S201–2.