To the Editor,
Lung cancer remains the leading cause of cancer-related mortality despite continuous efforts to identify effective treatments per se. The epidermal growth factor receptor (EGFR) inhibitors of tyrosine kinase (TKIs) are gaining increasing importance in the management of advanced non-small cell lung cancer (NSCLC). Through small-molecule tyrosine kinase inhibitors the approach of blocking the vascular endothelial growth factor receptor (VEGFR) tyrosine kinase has also demonstrated promise for several tumor types [Citation1].
Sunitinib is an oral, multitargeted inhibitor of multiple receptor tyrosine kinases (RTKs) including VEGFR-1, -2, and -3; PDGFR; KIT; and FLT-3, which drug confers both anti-proliferative and anti-angiogenic properties [Citation2]. This agent has been approved for treatment of imatinib-resistant or -intolerant gastrointestinal tumor (GIST) in addition to advanced and/or metastatic renal cell carcinoma (RCC). Presently it is being studied in various other solid tumor types. We reported that advanced NSCLC patients previously treated with multiregimens of chemotherapy and EGFR-TKI may still benefit from sunitinib treatment, who could develop tolerance to potential adverse events induced by sunitinib [Citation3]. Here, we describe one patient with lung cancer who experienced unexpectedly rapid progression and tumor related complaints after discontinuation of sunitinib.
Case report
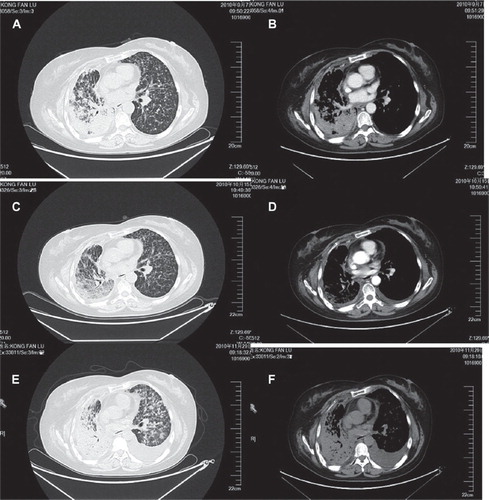
After having undergoing resection of a superior lobe of right lung and hilar lymphonode dissection in June 2007, followed by multiregimens of chemotherapy including such remedies as docetaxel plus carboplatin, pemetrexed plus cetuximab and gemcitabine, and EGFR-TKIs targeted therapy (Iressa and Tarceva), the 55-year-old non-smoking female patient was admitted to our hospital in September 2010, due to recrudescence and metastasis. The diagnosis made by pathological examination is moderately differentiated bronchioloalveolar carcinoma. After operation in June 2007 her disease was classified as stage IIIa according to pT2N2M0. She presented with such symptoms as dyspnea and cough in September 2010. The examination of chest computed tomography (CT)-scan revealed a right-sided soft tissue mass and multiple intrapulmonary metastases bilaterally (, ). Considering the patient's general condition and treatment history, we provided sunitinib as sixth line therapy, 37.5 mg daily orally for four weeks medication administration followed by two weeks discontinuation (Schedule 4/2) for a total of six-week cycle. According to Response Evaluation Criteria in Solid Tumors (RECIST), a partial remission was achieved after four-week treatment (, ). The symptoms of cough and dyspnea improved while on sunitinib treatment. Adverse events included grade 2 hand foot syndrome and mucositis based on (National Cancer Institute Common Toxicity Criteria; NCI-CTC). However, the symptoms of dyspnea and cough reappeared three days into the cycle 2 discontinuation period of sunitinib treatment. Despite the temporary alleviation of the hypoxia symptom following administration of dexamethasone, respiratory symptoms generally worsened with time. The chest CT-scan showed progression of the intrapulmonary metastases bilaterally and left-sided pleural effusion (, ).
Figure 1. Radiologic images before and after sunitinib 37.5 mg/d. A and B. Chest CT-scan of the thorax one week prior to sunitinib treatment (7 September 2010). C and D. Chest CT-scan of the thorax three days after one cycle of sunitinib treatment (15 October 2010). E and F. Chest CT-scan of the thorax one week after two cycles of sunitinib treatment (29 November 2010).
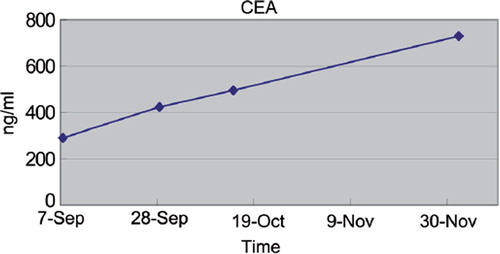
The patient's serum carcinoembryonic antigen (CEA) gradually increased from 289.9 ng/ml in September 2010 to 724.9 ng/ml in December 2010 () showing a generally accelerating tendency. Due to progressively worsening respiration failure, death occurred on 10 December 2010.
Discussion
A case of lung cancer patient was discussed with unexpectedly rapid clinical deterioration after stopping sunitinib. Sunitinib is an active agent in advanced NSCLC which displays high tolerance [Citation4]. The recommended dose of sunitinib is 50 mg daily orally for four weeks medication, administration followed by two weeks discontinuation. Considering the patient general condition and treatment history, we provided sunitinib at 37.5 mg daily orally as sixth-line therapy. One month after initial partial response (PR), she developed progressive disease (PD) with pleural effusion and multiple pulmonary metastases (, ). This flare up phenomenon is well recognized in daily clinical practice, although not well understood [Citation5].
Some oncologists attempted to explore plausible explanations and clinical consequences of the flare up phenomenon in renal cell cancer patients [Citation5,Citation6]. First of all, the effect of the angiogenesis inhibitors was considered to be fully exhausted when RECIST criteria for progressive disease are fulfilled. The residual inhibitory effect of the anti-angiogenic drug is removed when ceasing the therapy. In this respect, the usefulness of the RECIST criteria has raised doubts in anti-angiogenic treatment evaluation, owing to their cytostatic rather than cytotoxic effect. Subsequently, the mounting complaints after withdrawal of the angio-inhibitors is a rebound phenomenon regarding blood vessels. Blocking VEGF or VEGFR leads to apoptosis of endothelial cells and corresponding decrease in vascular diameters, density and permeability, which lowers interstitial fluid pressure and, in some tumors, elevates oxygen tension. Hypothetically, cessation of anti-angiogenic therapy leads to an increase of vascular density, tumor blood flow rate and permeability, which in turn cause extravasation of fluids, giving rise to reactive edema and pleural effusions. The complaints were noted shortly after cessation of therapy. The half-life of snitinib (including its active metabolite) is rather long (40–60 hours). However in mice, after cessation of sunitinib an increase of mean tumor volume was observed within 10 days, with plasma VEGF, placental growth factor (PlGF) and serum levels of soluble VEGFR-2 returning to baseline levels even faster. These data all suggest that despite the relatively long half-life of the drugs, the anti-tumor effects of angiogenesis inhibitors already alter within days after cessation. This also suggests an individual dose-effect response, which has not been subject of studies until now.
The patient in the description shows a clinically relevant flare up phenomenon. Identifying a resolution to this question is a major challenge in the anti-angiogenic treatment in NSCLC.
Declaration of interest: The authors report no conflicts of interest. The authors alone are respon-sible for the content and writing of the paper.
References
- Blumenschein G, Heymach JV. Angiogenesis inhibitors for lung cancer: Clinical developments and future directions. J Thorac Oncol 2006;1:744–8.
- Faivre S, Demetri G, Sargent W, Raymond E. Molecular basis for sunitinib efficacy and future trial development. Nat Rev Drug Discov 2007;6:734–45.
- Liu X, Wang W, Li J, Tang C, Yu F. Sunitinib in treatment of advanced non-small cell lung cancer after failure of multiregimens of chemotherapy and EGFR-TKI. J Clin Oncol 2010 ASCO Annual Meeting Proceedings 2010:e18139.
- Socinski MA. The current status and evolving role of sunitinib in non-small cell lung cancer. J Thorac Oncol 2008;3: S119–23.
- Wolter P, Beuselinck B, Pans S, Schöffski P. Flare up: An often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors. Acta Oncol 2009;4:621–4.
- Desar IM, Mulder SF, Stillebroer AB, van Spronsen DJ, van der Graaf WT, Mulders PF, . The reverse side of the victory: Flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients. A report of three cases. Acta Oncol 2009;6:927–31.