Abstract
Background. The relationship between Hashimoto's thyroiditis (HT) and papillary thyroid cancer (PTC) with regard to their co-occurrence and the effect of concurrent HT on the prognosis of PTC has been debated. The aim of the present study is to determine a clinical relationship between these two disease entities and to evaluate the correlation between concurrent HT and various clinicopathological parameters. Material and methods. Demographic and histopathological data were collected from 675 patients undergoing thyroid surgery from 2000 to 2005, and 303 patients who received initial treatment for thyroid disease at our institution and whose medical records were accessible for review were enrolled in this study. Of these participants, 269 with histologically confirmed PTC were analysed according to the presence or absence of concurrent HT. Results. Of 269 patients with PTC, 21.6% (58/269) had concurrent HT, whereas only 5.9% (2/34) had concurrent HT with another diagnosis (p = 0.031, odds ratio = 4.4; 95% CI, 1.02–18.90). Younger age at presentation and a greater female preponderance were noted in patients with HT compared with those without HT (p = 0.008 and p = 0.009, respectively). Although it was not statistically significant, PTC with HT patients tented to have smaller tumour size (1.6 ± 1.0 cm vs. 1.8 ± 1.5 cm), lower incidence of lymph node metastasis at presentation (12.2% vs. 29.9%), unifocal disease (84.5% vs. 78.7%), and early-stage disease. Additionally, PTC with HT patients exhibited better prognosis, viewed in recurrence and mortality, during the 62-month mean follow-up period. Discussion. HT was definitely associated with PTC as was chronic inflammation with cancer in other locations. Interestingly, however, the coexistence of HT in PTC cases introduced favourable clinical outcomes compared with those of PTC without HT.
Thyroid cancer is the most common head and neck malignancy in the US and accounts for approximately 1% of all cancers. In the US, it was estimated that 10 000 men, and 27 200 women would be diagnosed with thyroid cancer in 2009 [Citation1]. Papillary thyroid cancer (PTC), the most prevalent manifestation of thyroid cancer, represents 70–80% of all diagnosed thyroid cancers. In Korea, the prevalence of PTC is rapidly increasing, accounting for > 95% of all thyroid cancers [Citation2]. Among the inflammatory thyroid disease, Hashimoto's thyroiditis (HT), an autoimmune inflammatory disease characterised by widespread lymphocyte infiltration, fibrosis, and parenchymal atrophy [Citation3], is the most common cause of hypothyroidism in the US, affecting 22 of every 100 000 individuals [Citation4–6].
Since Virchow proposed in 1863 that cancer develops at a site of chronic inflammation [Citation7], chronic inflammation, leading to neoplastic transformation, has become a well-established clinical phenomenon. Chronic inflammatory reactions may result in cytokine production, which stimulates transformed cells growths. In some cases, chronic inflammation may increase the tissue stem cell pools, which then becomes subject to the effect of mutagens, and directly promotes genomic instability within cells through the production of reactive oxygen species. As described elsewhere, the relationship between HT and PTC was first proposed by Dailey et al. in 1955 [Citation8]. Since that initial report, the association between the two diseases has remained controversial, with various studies reporting up to a 38% concurrence rate [Citation5,Citation8–11]. Anecdotally, we have noticed an increased incidence of PTC associated with HT at our institution, and clinical implications between PTC and HT including the recurrence and long-term survival rate have been stronger than expected. However, few studies have focused on coexisting HT as a prognostic factor for associated PTC [Citation3,Citation5,Citation12]. Therefore, the aim of this study is to investigate PTC parameters and their relation to HT as well as the prognosis of PTC subdivided into tumours with and without coexisting HT. Because authors have assumed that it is important to study whether HT has an impact on PTC prognosis before studying whether it is a risk factor for PTC, we focused on the prognostic impact of concomitant HT in patients with PTC.
Material and methods
Patients
A database of patients with thyroid cancer was established upon inception of the Thyroid Cancer Center of Kyungpook National University Hospital in 1997. From these patients’ data, we could find that 675 patients underwent thyroid surgery at our institution in a six-year period of January 2000 to December 2005. Of these patients, 303 patients, who received initial treatment for thyroid disease at our institution and whose medical records were accessible for review were included in the study. The remaining patients were excluded due to inaccessible or incomplete medical charting, inappropriate pathologic slides for review of previous diagnosis, or revision surgery for recurrence. Patients who had been diagnosed with HT and who underwent regular examinations for any thyroid disease before being diagnosed with PTC were excluded to prevent selection bias, because they had more opportunities for early detection of PTC that could lead to better treatment outcomes.
The cohort of 303 patients enrolled in our present study consisted of 48 men and 255 women, with a mean age of 47.5 years at the time of surgery. Thyroid lobectomy with isthmusectomy was performed in 59 patients, whereas total thyroidectomy with or without neck dissection was performed in 244 patients. Central neck dissection (CND) without lateral compartment neck dissection was performed in 40 patients. Comprehensive neck dissections such as radical neck dissection (RND), modified RND (mRND), and selective neck dissection (SND) of level II to V including the central compartment were performed in 53 patients, and five of these patients underwent bilateral neck dissection.
Clinicopathological parameters
Patient data were collected by retrospective medical record review for patient demographics (age and gender) and histological reports including information about tumour size, extrathyroidal extension, multifocality, lymph node (LN) metastasis, and presence of HT. Clinical outcomes and progressions such as distant metastasis, recurrence, and death were also analysed. Pathologic staging was redefined according to the Tumor, Lymph Node, and Metastasis classification system based on the 6th edition of the UICC/AJCC TNM classifications. In addition, well-established prognosis classification systems of differentiated thyroid cancer, AMES stage and MACIS scoring system, were used in order to analyse various clinicopathological parameters [Citation13,Citation14]. AMES stage was divided into low-risk and high-risk group determined by Age, distant Metastasis, Extra-thyroidal extension, and tumour Size. MACIS scoring system is calculated with a formula using Metastasis, Age, Completeness of resection, extent of Invasion, and tumour Size. This system showed that high scores were strongly correlated to poor prognosis.
Review of histological diagnoses
Histological diagnoses were blindly determined by one co-author specialising in thyroid pathology (JY Park). The histological criteria used to make the diagnosis of HT included diffuse lymphoplasmacytic infiltration, germinal centres, and enlarged epithelial cells with large nuclei and eosinophilic cytoplasm (Askanazy or Hurthle cells). Non-specific lymphocytic thyroiditis occurring immediately adjacent to a tumour might represent perineoplastic inflammation and was not considered true HT to avoid over-diagnosis in our study. In addition, one co-author (JY Park) has reviewed all the pathological slides of 303 patients to reduce variations depending on pathologist in diagnoses for HT.
Treatment policy for thyroid cancer
Patients were recommended surgical treatment when the results of fine needle aspiration cytology of thyroid were consistent with malignant, or indeterminate such as follicular neoplasm, suspicion for malignancy, and atypia of follicular lesion. In addition, when patients complained of cosmetic problems due to huge thyroid nodule or goiter, surgery was also recommended regardless of the result of fine needle aspiration cytology.
All patients underwent surgery performed by the two experienced head and neck surgeons (JS Park, JH Sohn). The concrete principle of thyroid surgery was as follows. Thyroid lobectomy with isthmusectomy was performed only when the following criteria were met: the cancer was an intrathyroidal, unifocal microcarcinoma (≤1 cm), with no cervical lymph node involvement. In the other cases, total thyroidectomy was primarily performed. However, the authors were usually not concerned with patient factors such as age, sex, family history, or history of radiation. CND was performed when enlarged LN were seen in the surgical field of vision with 2.5 × loupes or detected by palpation of the central neck region. When metastasis to regional LN was identified in the preoperative evaluation or in follow-up examination after initial thyroid surgery, comprehensive neck dissection was performed from level II to VI. Radioactive iodine remnant ablation was performed using 100–150 mCi 131I with the same indications as those for total thyroidectomy.
Follow-up strategy
During the follow-up period, physical examinations were regularly performed on all patients every three to six months and plain chest radiographs were obtained once a year. For the patients given total thyroidectomy with or without neck dissection, first diagnostic whole body scans (WBS) with 150 mCi 131I and measurement of thyroglobulin (Tg) levels during thyroid hormone withdrawal (THW) were carried out 6–12 months after remnant ablation and then, diagnostic 131I WBS were routinely scheduled at one-year intervals. Serum Tg measurements, antithyroglobulin antibody (TgAb) assays, and TSH measurements were also performed at the time of the WBS. When serum Tg during THW was > 5 μg/l or there was clinical suspicion of recurrence, one or more non-radioiodine imaging methods, mainly neck ultrasonography (USG) and 18F-deoxyglucose (FDG) positron emission tomogram/computerised tomography (PET/CT), was performed to diagnose and localise recurrence.
For patients given thyroid lobectomy or less, a thyroid function test in addition to physical examination and chest radiography was performed to check the status of thyroid hormone (T3, free T4, and TSH), and thyroid USG covering the entire neck region was routinely scheduled every 6–12 months to evaluate recurrence in the contralateral thyroid and cervical lymph nodes.
Statistical analysis
SPSS for Windows (version 12.0; SPSS, Chicago, IL, USA) was used to analyse the data. Continuous data are represented as mean ± standard deviation. To compare continuous variables, age, tumour size, and mean MACIS scores according to HT status were tested using an independent t-test. The association between HT (present or absent) and prognostic variables was assessed using a χ2 test or Fisher's exact test for gender, extrathyroidal extension (present or absent), multifocality (unifocality or multifocality), LN metastasis (positive or negative), TNM and AMES stage, and MACIS scoring system. The Kaplan-Meyer method with a log-rank test was used to account for recurrence and survival rates during the follow-up period. With regard to the results, p-values were two-sided throughout and the statistical significance was defined as p < 0.05.
Results
Review of pathological diagnosis and HT frequency
In a pathological review of the 303 patients, 269 (88.8%) were diagnosed with PTC, followed by nodular hyperplasia and follicular thyroid cancer, in 11 (3.6%) patients and eight (3.0%) patients, respectively (). In addition, 60 (20.2%) total patients were identified as having pathological changes consistent with HT. In the subgroup of patients with HT, PTC was the most common comorbidity (58/60, 96.7%). That is, among 269 patients with PTC, 21.6% (58/269) had concurrent HT, whereas only 5.9% (2/34) had coexisting HT with other diagnoses, and there was a significant difference of 0.031 in p-value. Additionally, patients with PTC were about four times more likely to have HT compared with other diagnoses (odds ratio = 4.4; 95% CI, 1.02 –18.90).
Table I. Permanent histological diagnosis of 303 patients.
Baseline clinicodemographic variables
Patients with PTC and HT appeared to be slightly younger than those without HT (mean age 42.8 ± 12.7 vs. 48.3 ± 14.4); this difference was statistically significant (p = 0.008). In addition, Patients with PTC and HT had a greater than four times female predominance compared with that of patients with PTC alone; the female to male ratios were 18.3:1 vs. 4.2:1, respectively (p = 0.009). There was no significant difference between the two groups with respect to the extent of thyroid surgery. However, in terms of the neck dissection, CND without lateral neck dissection was more frequently performed in patients with PTC and HT than in patients with PTC alone (22.4 vs. 10.9%; p = 0.023), while comprehensive neck dissection was more prevalent in patients with PTC alone than in patients with PTC and HT (20.9 vs. 8.6%; p = 0.033). The duration of hospital stay was significantly longer in patients with PTC alone (7.1 ± 6.4 vs. 5.1 ± 2.9 days; p = 0.001). The incidence of major surgical complications, such as vocal fold palsy and hypocalcemia, was not statistically different between the two groups ().
Table II. Baseline clinicodemographic variables of 269 patients with papillary thyroid cancer (PTC) stratified by the presence of Hashimoto's thyroiditis (HT).
Univariate clinicopathological parameters
lists the univariate clinicopathological factors stratified by the presence or absence of HT in patients with PTC. Patients with PTC and HT were similar to patients with PTC alone with regard to sensitivity of fine needle aspiration cytology (91.4% vs. 87.7%; p = 0.251), proportion of conventional PTC (94.8% vs. 96.2%; p = 0.638), mean tumour size (1.6 cm vs. 1.8 cm; p = 0.368), multifocality (15.5% vs. 21.3%; p = 0.328), extrathyroidal extension (62.1% vs. 59.2%; p = 0.697), and TNM stages. Although a considerably lower percentage of LN metastasis (12.2% vs. 29.9%; p = 0.056) at the present was found in patients with PTC and HT compared with patients with PTC only, the statistical significance was not verified.
Table III. Univariate clinicopathological parameters of 269 patients with papillary thyroid cancer (PTC) stratified by the presence of Hashimoto's Thyroiditis (HT).
Prognosis evaluating system
Evaluation of the expectant prognostic outcome using the AMES clinical staging system [Citation13] and the MCIS scoring system [Citation14] was also performed. With the AMES staging system, proportion of high risk group of patients with PTC alone was slightly higher than that of patients with PTC and HT (29.4% and 24.1%, respectively; p = 0.432). Using the MACIS scoring system, this trend between the two groups became more evident. Patients with PTC alone exhibited a higher mean score (5.49 vs. 4.98; p = 0.03), and a higher percentage (31.3% vs. 20.7%) of these patients scored 6 or higher on the MCIS scoring system; however, this difference was not statistically significant (p = 0.115) ().
Table IV. AMES stage and MACIS score in patients with papillary thyroid cancer (PTC) and Hashimoto's thyroiditis (HT) and in patients with PTC alone.
Diagnosis of recurrence and result of survival
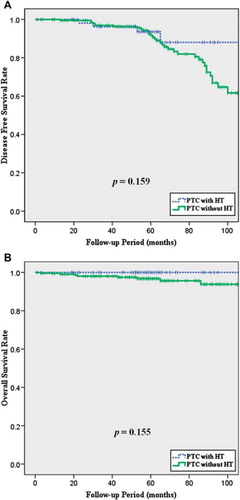
Patients were evaluated by physical examination, USG, and/or PET/CT to identify recurrence, and all patients in whom recurrence was suspected were confirmed using cytology and/or histology. During the mean 59-month follow-up period in the PTC with HT group, four (6.9%) of 58 patients experienced recurrence and all of the recurrent diseases occurred at the regional LN. Comprehensive neck dissection followed by high-dose radioactive iodine remnant ablation therapy was performed in all of these patients, and none have died at the time of the present evaluation. Of the patients with PTC only with a mean 62-month follow-up period, a total of 35 (16.6%) have experienced recurrence, the main location also being the regional LN (29 patients; 82.9%). Overall, 8 (3.8%) patients with PTC alone have died, but only two (1.0%) of these deaths were due to PTC or related complications. Interestingly, both patients died of PTC derived-anaplastic thyroid cancer. Patients with PTC and HT tended to have a better prognosis compared with that of patients with PTC alone, but the differences between the two groups were not statistically significant in terms of disease-free or overall survival rates during mean 60-month follow-up (p = 0.159 and p = 0.155, respectively; (, ).
Table V. Recurrence and mortality in patients with papillary thyroid cancer (PTC) and Hashimoto's thyroiditis (HT) and in patients with PTC alone.
Discussion
Chronic inflammation is increasingly recognised as playing a major role in the development of malignant tumors from multiple sites. In the thyroid gland, however, the association between PTC and HT remains unclear. Crile found only one case of PTC in a population-based study of 200 patients with HT for > 1000 patient-years of follow-up duration [Citation10], and Holm showed an increased association only between HT and lymphoma [Citation9]. The mainstream of recently reported studies showed a higher incidence of PTC in patients with HT and/or a higher prevalence of HT in patients with PTC [Citation3,Citation12,Citation15–18]. In the current study, the authors identified a 21.5% incidence of HT associated with PTC, consistent with recent reports of 12–43.8%. A statistically significant odds ratio of 4.4 was also found, which is considerably higher than reported in other studies [Citation3,Citation5,Citation9–11,Citation16,Citation19]. In addition, PTC coexisting with HT was more likely to occur in much younger patients and have a greater female predominance. Singh et al. [Citation5] and Loh et al. [Citation20] reported that PTC patients with HT were two or three years younger than those without HT, and Repplinger et al. [Citation17] reported that women with HT were 30% more likely to have coexisting PTC compared with other females without HT.
However, the large variation in incidence between studies likely suggests that there is a significant difference in the pathological definition and interpretation of HT depending on institutions and pathologists. Nonspecific peritumoral infiltration that reflects an immune response to a tumour in particular should be excluded from true HT groups in order to evaluate the precise incidence of HT in PTC.
Because the clinical relationship between HT and PTC remains unclear, a number of proposed mechanisms for both of these diseases exist in the literature. McConahet et al. [Citation21] described in 1972 chronic stimulation of the thyroid by elevated TSH levels may initiate or promote the growth of thyroid neoplasm, and Wirtschafter et al. [Citation22] identified expression of the RET/PTC1 and RET/PTC3 oncogenes in HT patients. Most recently, Larson et al. [Citation16] reported increased PI3K/Akt expression in both HT and well-differentiated thyroid cancers, suggesting a possible molecular mechanism of thyroid carcinogenesis.
Emerging literature suggests a protective effect of HT in patients with PTC. In a large retrospective study, Kashima et al. [Citation19] reported a 0.7% cancer specific mortality and a 95% relapse-free 10-year survival rate in patients with chronic thyroiditis compared to a 5% mortality and 85% relapse-free 10-year survival rate without chronic thyroiditis; these findings were similar to Loh's results [Citation20]. Singh et al. [Citation5] identified a positive correlation between HT in patients with PTC and disease-free survival and overall survival; hence, they concluded that patients with PTC in the presence of HT had more a favourable clinical outcome in terms of recurrence and mortality. Most recently, Kim et al. [Citation12] studied the clinical impact of coexisting chronic lymphocytic thyroiditis and found that it was associated with smaller primary tumour size at presentation and a reduced risk of recurrence, although this difference was not significant after adjustment was made for other prognostic factors. In our study, coexisting HT was assumed to be associated with better prognostic factors, such as smaller tumour size, lower incidence of LN metastasis, unifocal disease, and lower clinical stage and MACIS score at the time of initial surgery than those of patients with PTC alone. In addition, patients with PTC and HT tend to have a more indolent clinical course compared to those with PTC alone, including lower recurrence rates and higher overall survival rates; however, the statistical significance of this finding was not verified. The lack of significance in these prognostic parameters is most likely due to small sample size and insufficient follow-up duration. Furthermore, because overall prognosis of PTC is remarkably excellent itself, it is difficult to analyse survival differences between subgroups of PTC. Although the p-values did not meet our criteria for statistical significance, these results would possibly change with additional patients and an increased follow-up period.
Hypotheses of the mechanism of a better prognosis in PTC with HT have been evaluated in different ways. Giodarno et al. [Citation23] reported that follicular cells in HT express both Fas and Fas ligand that probably activate the Fas-mediated apoptotic pathway resulting in destruction of the thyroid tissues. HT is a kind of autoimmune disease that leads to the destruction of thyroid follicles through an immune response to a thyroid specific antigen. As PTC cells originating from the follicular cells would express the thyroid specific antigen, auto-antibodies from coexisting HT might destroy the tumour cells in much the same way as in HT alone [Citation12]. Additionally, the infiltrated lymphocytes in patients with PTC are likely to be cytotoxic T cells acting as carcinoma cell killers, secreting interleukin-1 that inhibits thyroid cancer cell growth [Citation24]. In studies on BRAFV600E, a known factor of an aggressive course for PTC, Kim et al. [Citation25] reported a significantly lower prevalence of BRAFV600E mutation in patients with PTC and HT, suggesting that HT is less likely to be associated with poor prognostic outcomes.
In conclusion, we have shown a clinical relationship between two disease entities. Patients with PTC were four times more likely to demonstrate a coexisting HT compared with patients with other thyroid disease, suggesting a link between chronic inflammation and cancer development in the thyroid gland. There was also a trend in patients with PTC and HT for a better prognosis: smaller tumour size, lower incidence of LN metastasis, lower MACIS score, and higher disease-free and overall survival rates than in patients with PTC alone, although these findings were not statistically significant.
However, the present study has some potential limitations. The study was based on patients undergoing thyroid surgery; this would undoubtedly introduce a selection bias into this study population since thyroid surgery in the author's institution was performed mostly when the nodule was suspected of malignancy. Moreover, since many patients with HT are not treated surgically, we are unable to account for the true incidence of HT. A prospective cohort study of the general population should be performed in the future to overcome these limitations.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- Tang KT, Lee CH. BRAF mutation in papillary thyroid carcinoma: Pathogenic role and clinical implications. J Chin Med Assoc. 2010;73:113–28.
- Kwak JY, Kim EK, Kim JK, Han JH, Hong SW, Park TS, . Dual priming oligonucleotide-based multiplex PCR analysis for detection of BRAFV600E mutation in FNAB samples of thyroid nodules in BRAFV600E mutation-prevalent area. Head Neck. 2010;32:490–8.
- Cipolla C, Sandonato L, Graceffa G, Fricano S, Torcivia A, Vieni S, . Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. Am Surg. 2005;71:874–8.
- Lal G, Clark OH. Chronic thyroiditis. Textbook of endocrine: Surgery. Philadelphia: Saunders; 2005.
- Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: Impact on presentation, management, and outcome. Surgery. 1999;126:1070–6; discussion 1076–7.
- Kumar V, Robbins SL. The endocrine system. Robbins basic pathology. Philadelphia: Saunders/Elsevier; 2007.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7.
- Dailey M, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291–7.
- Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601–4.
- Crile GJ. Struma lymphomatosa and carcinoma of the thyroid. Surg Gynecol Obstet. 1978;147:350–2.
- Shands WC. Carcinoma of the thyroid in association with struma lymphomatosa (Hashimoto’s disease). Ann Surg. 1960;151:675–82.
- Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, . Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009;71: 581–6.
- Sanders LE, Cady B. Differentiated thyroid cancer: Reexamination of risk groups and outcome of treatment. Arch Surg. 1998;133:419–25.
- Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: Development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–7.
- Shim YS, Lee YS, Lee GH, Lee BC, Jung YW, Lee JW, . Clinical assessment and management of papillary thyroid carcinoma with coexistent Hashimoto’s thyroiditis. Korean J Otolaryngol – Head Neck Surg. 2007;50:537–1.
- Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, . Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007;204:764–73.
- Repplinger D, Bargren A, Zhang YW, Adler JT, Haymart M, Chen H. Is Hashimoto’s thyroiditis a risk factor for papillary thyroid cancer? J Surg Res. 2008;150:49–52.
- Pisanu A, Piu S, Cois A, Uccheddu A. Coexisting Hashimoto’s thyroiditis with differentiated thyroid cancer and benign thyroid diseases: Indications for thyroidectomy. Chir Ital. 2003;55:365–72.
- Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, . Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998;8:197–202.
- Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:458–63.
- McConahey WM. Hashimoto’s thyroiditis. Med Clin North Am. 1972;56:885–96.
- Wirtschafter A, Schmidt R, Rosen D, Kundu N, Santoro M, Fusco A, . Expression of the RET/PTC fusion gene as a marker for papillary carcinoma in Hashimoto’s thyroiditis. Laryngoscope. 1997;107:95–100.
- Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, . Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science. 1997;275:960–3.
- Kimura H, Yamashita S, Namba H, Tominaga T, Tsuruta M, Yokoyama N, . Interleukin-1 inhibits human thyroid carcinoma cell growth. J Clin Endocrinol Metab. 1992;75: 596–602.
- Kim SK, Song KH, Lim SD, Lim YC, Yoo YB, Kim JS, . Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditis. Thyroid. 2009;19:137–41.