Abstract
Background and purpose. Loco-regional radiotherapy of left-sided breast cancer represents a treatment planning challenge when the internal mammary chain (IMC) lymph nodes are included in the target volume. This treatment planning study evaluates the reduction in cardiopulmonary doses when radiation is given during deep inspiration breath-hold (DIBH). This was achieved without compromising dose coverage to the planning target volume (PTV). Patients and methods. Seventeen patients with early breast cancer, referred for adjuvant radiotherapy, were included. For each patient two computed tomography (CT)-scans were acquired; the first during free breathing (FB) and the second during DIBH. The scans were monitored by the Varian RPMTM respiratory gating system. Audio-visual guidance was used. The treatment planning of the two CT studies was performed focusing on good coverage (V95% > 98%) of the PTV. Doses to the heart, left anterior descending (LAD) coronary artery, lungs and contralateral breast were assessed. Results. With equal PTV coverage, average mean heart dose was reduced from 6.2 Gy to 3.1 Gy in DIBH plans as compared to FB. Average volume receiving 25 Gy or more (V25Gy) was reduced from 6.7% to 1.2%, and the number of patients with V25Gy > 5% was reduced from 8 to 1 utilizing DIBH. The average mean dose to the LAD coronary artery was reduced from 25.0 Gy to 10.9 Gy. The average ipsilateral lung volume receiving 20 Gy or more (V20Gy) was reduced from 44.5% to 32.7% with DIBH. In 11 of the DIBH plans V20Gy was lower than 35%, in accordance with national guidelines, while none of the FB plans fulfilled this recommendation. Conclusion. Respiratory gated radiotherapy during DIBH is a suitable technique for loco-regional breast irradiation even when IMC lymph nodes are included in the PTV. Cardiopulmonary doses are considerably decreased for all dose levels without compromising the dose coverage to PTV.
Loco-regional radiotherapy (RT) of left-sided breast cancer represents a treatment planning challenge, especially when the internal mammary chain (IMC) lymph nodes are included in the target volume. Due to the increased radiation dose, especially to the heart, the IMC lymph nodes are often excluded from the target volume. However, recent studies indicate increased survival after radiation of IMC lymph nodes among patients with lymphatic draining to the IMC lymph nodes [Citation1,Citation2]. Together with the extended use of cardiotoxic systemic therapy this highlights the need for improvements in RT delivery to reduce irradiation to organs at risk (OAR) and normal tissue. In recent years different RT techniques, such as intensity modulated RT (IMRT), volumetric modulated arc therapy (VMAT), tomotherapy and respiratory gating have been introduced as possible treatment strategies to reduce the cardiopulmonary doses.
Several authors [Citation3–5] have shown that respiratory gating reduces the cardiopulmonary doses when irradiating during deep inspiration. Deep inspiration increases the distance between the breast and the heart due to increased lung volume, and the heart is partially or completely moved out of the high dose region. The relative volume of the ipsilateral lung exposed to irradiation is also decreased. In a recent treatment planning study we were able to show that radiation during deep inspiration breath-hold (DIBH), utilizing audio-visual guidance of the patient, allows tangential treatment of left-sided breast cancer patients with considerably reduced cardiac doses without compromising target coverage [Citation6].
The purpose of this treatment planning study was to carefully evaluate the dose to OAR for patients with left-sided breast cancer when irradiating the breast and loco-regional lymph nodes in the axilla, supraclavicular region and IMC during free breathing (FB) and DIBH, respectively. The inclusion of axillary levels, supraclavicular region and the IMC was done for scientific purposes only in order to test our DIBH technique in a “worst case” scenario. To our knowledge, this is the first study with a reasonable number of patients that documents good and similar dose coverage to the planning target volume (PTV) for both techniques to be compared. Equal PTV dose coverage is important when comparing the dose to OAR. In contrast to others who have used the DIBH technique, we gave our patients audio-visual guidance to reach the same amplitude level at each deep breath, and to maintain very stable amplitude within each breath-hold.
Patients and methods
Patient population
Computed tomography (CT) series of 17 patients who were referred for adjuvant RT after breast conserving surgery at Stavanger University Hospital between January and October 2006 were analyzed. The inclusion of patients was not consecutive, but based on logistics availability (staff and venues). Twelve of these patients had left-sided and five had right-sided breast cancer, but for study purposes the mammary glandular tissue and lymph nodes in the axilla, supraclavicular region and the IMC of the left side were defined as the target in all patients. The median age was 60 (range: 29–70) years. The patients had to be able to cooperate and to hold their breath for 15–20 s. The study was approved by the regional ethical committee as a project for quality assurance in health care. The CT series were taken prior to the clinical implementation of the DIBH technique, and with one exception, the patients were not treated with respiratory gating.
CT scanning
Two CT series were obtained for each patient, one during FB and the other during DIBH. The patients were placed in a supine position with bilateral arm abduction above the head using a PosiboardTM-2 breast board (CIVCO Medical Solutions, Kalona, IA, USA). In our department all breast cancer patients undergoing RT are placed in this treatment position, independent of laterality and lymph node involvement. CT-scans were performed at 3 mm intervals and encompassed both breast and the whole thoracic cavity including the heart and both lungs. The image acquisition was in helical mode.
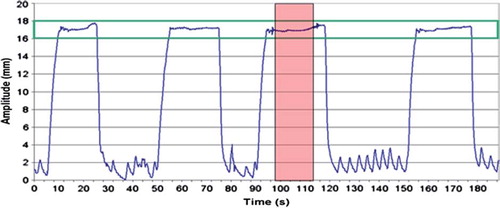
The Varian RPMTM respiratory gating system, version 1.6 (Varian Medical Systems, Palo Alto, CA, USA) was used for real time registration of the respiration. An infrared reflecting marker was placed on the patient, normally over the xiphoid process, and a video camera registered the anteroposterior motion of the marker due to respiration. The amplitude of DIBH, i.e. the chest wall motion was individually set prior to the CT-scan. The DIBH amplitude was only allowed to deviate ± 1 mm during the scanning. shows a typical DIBH breathing curve. The patient received visual guidance through the binocular head mounted display to sustain reproducibility and stability of the DIBH amplitude. The scanning time was typically around 20 s and most patients managed to complete the scan during one DIBH. For those who did not, the CT had to be manually stopped, and restarted during the next DIBH. In this study the mean anteroposterior (AP) chest wall movement at the position of the xiphoid process was 3 mm (range: 1.6–5.0 mm) during FB. With the DIBH technique the chest wall was moving an average of 18 mm (range: 14.6–27.0 mm) in the anterior direction when the patients breathed in deeply, and during breath hold the AP chest wall movement was only allowed to vary 2 mm.
Figure 1. A typical DIBH breathing curve from CT-scanning, with gaps of FB between each DIBH. The CT-scan was acquired during one DIBH (pink area) and the gating window (green lines) was set to the mean amplitude ± 1 mm. CT, computed tomography; DIBH, deep inspiration breath-hold; FB, free breathing.
Delineation of target and OAR
The CT series were transferred to an Eclipse 3-D treatment planning workstation, version 8.0 (Varian Medical Systems) and the clinical target volume (CTV) and OAR were delineated.
The mammary glandular tissue and lymph nodes in the axilla, supraclavicular region and in the IMC of the left side were defined as CTV. The internal mammary nodes located in intercostal space 1 to 3 and nodes of level I, II and III of the axilla were completely included in the CTV.
The heart, the left anterior descending (LAD) coronary artery, both lungs and the contralateral breast were considered OAR. The lung volume was automatically generated using the auto-contouring tool of the treatment planning system. The heart volume was defined as the entire visible myocardium, including the pericardium, from apex of the heart, to the right auricle, atrium, and infundibulum of the ventricle. The ascending aorta, the pulmonary trunk and the superior vena cava were excluded. The LAD coronary artery was delineated in the anterior interventricular groove to the apex of the heart. The contralateral breast was defined as all glandular breast tissue of the right side.
For consistency, the delineation of CTV and the contralateral breast was performed by the same oncologist for all patients. The LAD coronary artery and the heart were delineated by a radiation technologist. Delineation of the LAD coronary artery was done under supervision from a cardiologist, and the delineation of the heart was verified by the oncologist.
The margin from CTV to PTV was 5 mm, except for superficial areas where PTV was never closer than 5 mm to the skin. The margins were the same for DIBH and FB. Due to the chosen 2 mm gating window the movement of the chest wall during the DIBH plateau was the same, or smaller, as compared to FB. Hence the margin to compensate for CTV motion did not have to be increased with the DIBH technique. OAR were delineated without margins.
Treatment planning
An individually optimized mono-isocentric photon field treatment plan was obtained for each CT series. The technique included wide opposing 6 MV tangential fields covering the remaining breast and internal mammary nodes and AP-PA fields, 6 MV and 15 MV, respectively, covering the axillary and supraclaviculary nodes, abutting each other half-beam. Occasionally low weighted 15 MV fields, with the same geometry as the 6 MV tangential fields, were added. All fields were conformal with multileaf collimators and wedges in some cases. The isocenter was individually determined, but was equally positioned inside the CTV in the transition between mammary tissue and supraclavicular lymph nodes in both CT series, FB and DIBH, for the same patient.
The following dose constraints were given:
1) Minimum 98% of the PTV should be covered by the 95% isodose line (V95% ≥98%).
2) The 95% isodose line should cover the dorsal limit of the PTV (by visual inspection).
3) The mean dose to PTV should be close to 100% of the prescribed dose, and not above 102%.
4) Dose maximum should not exceed 110% and preferably not 107%.
5) The dose to OAR should be kept as low as possible, without compromising the PTV dose, i.e. the criteria of PTV coverage should be fulfilled, even if the national guidelines for doses to OAR were exceeded.
For each patient only minor difference between the two plans, FB and DIBH, with respect to target dose coverage, dose conformity, maximum dose, beam energy and geometry, should be accepted.
In a clinical setting the prescribed dose to PTV is 46 Gy in 2 Gy fractions followed by 4 Gy in 2 Gy fractions to the left breast only. For simplicity, in this study the prescription dose was 50 Gy in 2 Gy fractions to PTV. The dose to heart and ipsilateral lung is therefore slightly increased, about 0.5%, as compared to the clinical setting.
National guidelines recommend the following dose limitation to OAR:
1) The volume of the heart receiving a dose of 25 Gy or more should be smaller than 5% (V25Gy ≤ 5%).
2) The volume of ipsilateral lung receiving a dose of 20 Gy or more should be smaller than 35% (V20Gy ≤ 35%).
The guidelines do not have specific recommendations regarding the dose to contralateral breast and LAD coronary artery, except keeping them as low as possible.
The calculation algorithm used was Eclipse pencil beam (PB) described by Storchi et al. [Citation7] with the modified Batho inhomogeneity correction [Citation8]. All plans were made by the same physicist.
Outcome measures and statistical analysis
For each patient cumulative dose-volume histograms (DVHs) were calculated for all delineated volumes in the two different treatment plans. Dose to PTV and OAR was assessed. The volumes, mean and maximum doses were obtained from the DVH statistics. The relative volume Vx, irradiated to a minimum dose x (in Gy or %), e.g. V25Gy for the heart, V20Gy for the ipsilateral lung and V95% for PTV, were obtained from the DVH graphs. The relative dose Dx%, covering minimum x% of the volume of interest, e.g. D2%, D50% and D98% were also obtained from the DVH graphs. The maximum heart distance (MHD) and maximum lung distance (MLD) were measured in beams eye view.
The homogeneity and conformity indices were calculated. The homogeneity index (HI) for PTV is defined as follows [Citation9]:
HI = (D2%–D98%)/D50%
An HI of zero indicates that the dose distribution is almost homogeneous.
The target conformity index (CI) as well as the healthy tissues conformity index (CIHealthy Tissues) were calculated. The CI is defined as follows [Citation10]:
CI = (V95%/VPTV)
where V95% is the volume of PTV covered by the 95% isodose line, and VPTV is the volume of PTV. This equation defines the quality of the coverage of the target. The index ranges from 0 to 1, where 1 indicates that all of the target volume is covered by the prescribed dose.
The healthy tissues conformity index is defined as follows [Citation10], when the reference isodose is set equal to the 95% isodose:
CIHealthy Tissues = (V95%/TV95%)
where TV95% is the volume of the 95% isodose. This equation defines volume of healthy tissue receiving a dose greater than or equal to 95% of the prescribed dose. The CIHealthy Tissues ranges from 0 to 1, where 1 is the ideal value (perfect conformation).
The distance from isocenter to the cranial limit of CTV was obtained from the CT series, by counting the number of CT slices from isocenter to the last cranial slice with CTV delineation, and multiply by the slice thickness of 3 mm. Similarly, the distances from isocenter to the cranial limit of the lung and the mammary tissue of the left side as well as the caudal limit of CTV in the IMC region were obtained.
Paired Wilcoxon test was used for statistical analysis of the differences with computer software SPSS version 16.0. Data were considered statistically significant for p < 0.05.
Results
PTV and OAR volumes
shows the mean volume of PTV and OAR during FB and DIBH for all 17 patients. Compared to FB, the mean volume of ipsilateral lung increased about two-fold during DIBH (1178.2 cm3 vs. 2165.1 cm3, p < 0.001), while the mean heart volume decreased about 10% in size (616.5 cm3 vs. 559.8 cm3, p = 0.003). For the other delineated volumes no significant difference in size was found.
Table I. Mean volume, in cm3, for planning target volume (PTV) and organs at risk (OAR) during free breathing (FB) and deep inspiration breath-hold (DIBH) for all 17 patients. Data are shown as mean values with one standard deviation, and range in brackets.
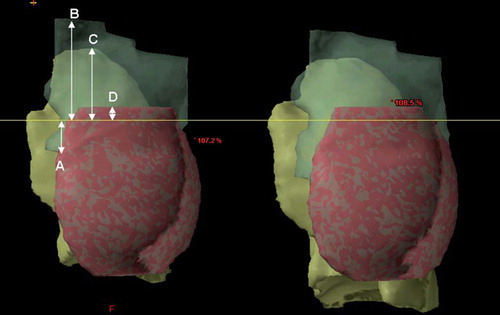
shows the CTV, the mammary tissue and the ipsilateral lung for the same patient during FB and DIBH. The position of the isocenter inside the CTV was identical for the two CT series, 1.24 cm caudally to the cranial border of the mammary tissue. As shown in the shape and position of CTV was somewhat changed during DIBH compared to FB. The CTV was moved cranially during DIBH with the isocenter position (mean: 0.82 cm) closer to the apex of the lung. During DIBH the length of the supraclavicular region was reduced with 1.12 cm (mean value, p < 0.001) whereas the length of the IMC region was increased with 0.67 cm (p = 0.042) as compared to FB.
Figure 2. CTV (translucent green), mammary tissue (pink) and ipsilateral lung (yellow) for the same patient during FB (left) and DIBH (right). The yellow line shows the craniocaudal position of the isocenter. The white arrows show the measured distances from isocenter to the caudal limit of the internal mammary nodes (A), from isocenter to the cranial limit of CTV (B), from isocenter to cranial limit of the ipsilateral lung (C), as well as the distance from isocenter to the cranial limit of the mammary tissue (D). CTV, clinical target volume; DIBH, deep inspiration breath-hold; FB, free breathing.
Treatment planning and PTV coverage
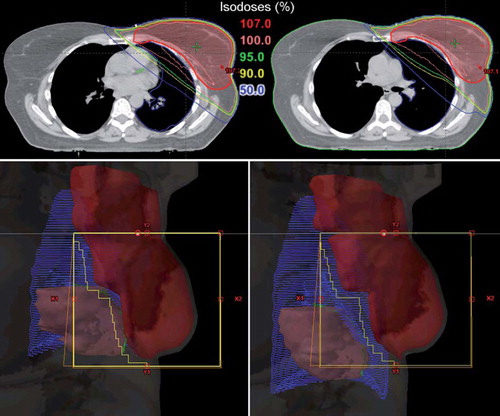
The dose distribution and a beam's eye view of the medial tangential field for both plans for the same patient are shown in . The figure shows that the lungs greatly increase in volume during deep inspiration, separating the heart from the chest wall. In addition, the heart moves caudally as the lungs push the diaphragm downward. Thus, the heart is moved out of the beam portals and the high dose region.
Figure 3. The upper panel shows the dose distribution in a transversal CT slice obtained at the same position of PTV for the same patient during FB (left panel) and DIBH (right panel), respectively. The heart moves out of the high dose region during DIBH. The lower panel shows beam's eye views of the medial tangential field during FB (left) and DIBH (right) for the same patient as upper panel. During inspiration the lung volume (blue) is increased, the breast (red) is moved cranioventrally and the heart (pink) caudally. In the shown case, the heart and the LAD coronary artery (green) was not included in the beam portal (yellow lines) during DIBH. CT, computed tomography; DIBH, deep inspiration breath-hold; FB, free breathing; LAD, left anterior descending; PTV, planning target volume.
The mean DVHs for FB and DIBH, averaged for all 17 patients, for PTV are shown in . The DVHs for PTV were quite similar in DIBH and FB plans reflecting a similar and homogeneous PTV coverage. The treatment planning results are summarized in . All plans fulfilled the criterion of dose coverage to the PTV as described in methods. There was no difference in average mean dose, D98% and V95% for PTV in FB and DIBH, whereas average D2% was slightly higher in the DIBH plans (53.2 Gy vs. 53.0 Gy in FB plans, p = 0.006).
Figure 4. Mean DVHs averaged for all 17 patients for PTV and OAR with FB (solid line) and DIBH (dotted line). DIBH, deep inspiration breath-hold; DVH, dose-volume histograms; FB, free breathing; OAR, organs at risk; PTV, planning target volume.
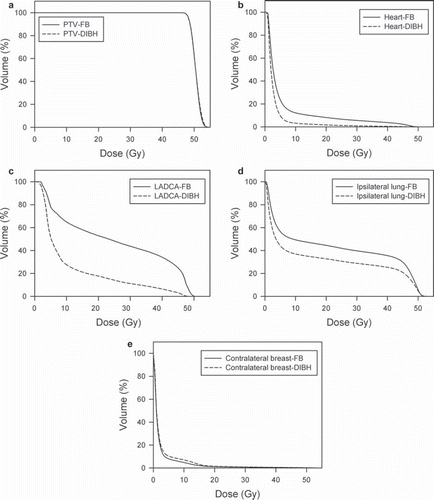
Table II. Summary of treatment planning data for PTV and OAR for the 17 breast cancer patients included in this study, with FB and DIBH. The prescription dose was 50 Gy in 2 Gy fractions. For comparison with earlier studies maximum doses are also shown.
No statistical differences were seen in mean HI and mean target CI in FB and DIBH plans. The CIHealthy Tissue, however, were significantly lower in the DIBH plans (0.62 vs. 0.64 in FB plans, p = 0.006).
Organs at risk
The mean DVHs for FB and DIBH, averaged for all 17 patients, for the OAR, are shown in . The mean DVHs show that the low-, medium- as well as the high dose region in the heart, the LAD coronary artery and the ipsilateral lung were reduced with DIBH. For the contralateral breast the mean DVHs indicate that the volumes of low and medium doses were slightly increased with the DIBH technique. The OAR treatment planning results are listed in .
Cardiac doses
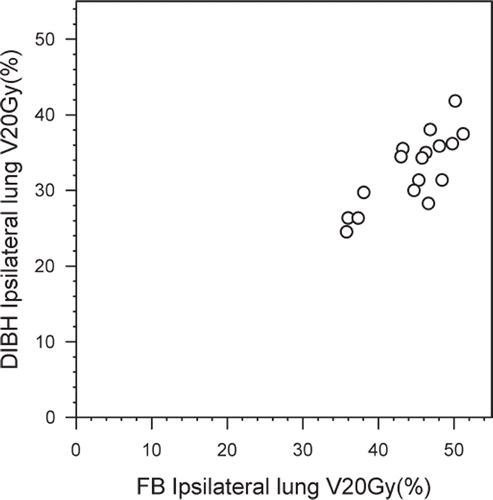
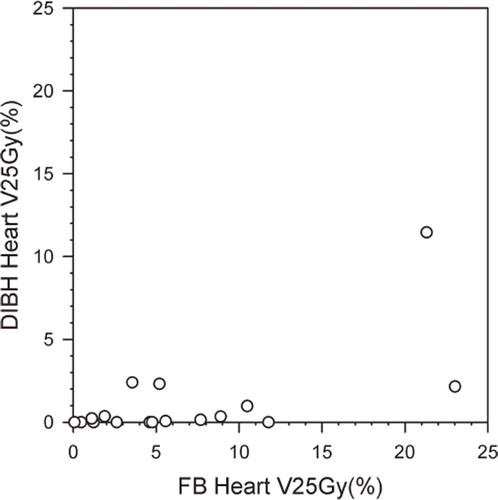
All treatment plans based on FB technique included heart tissue within the beam portals (). With the DIBH technique the heart was completely outside the beam portals for seven of the 17 patients (41.2%). The average MHD decreased from 1.9 cm (range 0.9–3.7 cm) using FB to 0.7 cm (range 0.0–3.4 cm) using DIBH. The average mean heart dose was reduced from 6.2 Gy (range 2.5–14.4 Gy) to 3.1 Gy (range 1.8–9.7 Gy) with the DIBH technique. Both of these differences were significant (p < 0.001). The mean V25Gy was significantly decreased in the DIBH plans (1.2% vs.6.7% with FB, p < 0.001). Scatter plot of V25Gy in FB and DIBH plans for each patient are shown in . V25Gy was larger than 5% in eight patients (47.1%) using the FB technique, compared to only one patient (5.9%) when using DIBH (). The largest V25Gy value observed was 23.0% for FB and 11.5% for DIBH. These values were not from the same patient. The patient with the largest V25Gy value (23%) during FB achieved a V25Gy of 2.2% with the DIBH technique. Average D2% for the heart was reduced from 34.1 Gy in FB plans to 13.1 Gy in DIBH plans (p < 0.001).
Figure 5. Volume of heart (in %) receiving 25 Gy or more (V25Gy) in FB plans versus DIBH plans plotted for each patient individually. DIBH, deep inspiration breath-hold; FB, free breathing.
For the LAD coronary artery there was a significant (p < 0.001) reduction in average mean dose from 23.0 Gy (range 3.7–48.2 Gy) to 10.9 Gy (range 3.1–38.9 Gy). Mean V25Gy for the LAD coronary artery was also significantly reduced, from 48.4% with FB to 14.1% with DIBH (p = 0.001). Average D2% for the LAD coronary artery was reduced (p < 0.001) from 39.2 Gy (range 5.1–50.1 Gy) to 24.1 Gy (range 4.0–47.8 Gy).
Pulmonary doses
Average mean dose to the ipsilateral lung was 5.3 Gy lower with DIBH than with FB (16.4 Gy vs. 21.7 Gy, p < 0.001). Similarly, the average V20Gy for ipsilateral lung was significantly reduced from 44.5% (range 35.8–51.2%) using FB to 32.7% (range 24.5–41.8%) using DIBH (). V20Gy for each patient individually, FB versus DIBH, is plotted in . All patients had V20Gy higher than 35% with FB, whereas this was true only for six of the 17 patients with DIBH (). In FB, 13 patients had V20Gy > 41.8% which was the largest V20Gy value observed in DIBH plans. The average MLD of the medial field increased from 4.7 cm with FB to 5.1 cm with DIBH (p = 0.017). Average mean dose to total lung volume was reduced with 2.2 Gy using DIBH, from 10.3 Gy (range 8.4–12.1 Gy) with FB to 8.1 Gy (range 6.5–10.7 Gy) with DIBH (p < 0.001).
Contralateral breast
Average mean dose to the contralateral breast was 0.5 Gy higher in DIBH plans than in FB plans (2.7 Gy vs. 2.2 Gy, p = 0.012), and V2Gy was 3.9% larger (26.1% in DIBH plans vs. 22.2% in FB plans, p = 0.049). Average D2% was also slightly increased in the DIBH plans (16.5 Gy vs. 13.2 Gy with FB, p = 0.047).
Discussion
In this CT planning study we were able to demonstrate that irradiation of the left breast and regional lymph nodes, including the IMC, in a DIBH technique utilizing audio-visual guidance, leads to a substantial reduction in cardiopulmonary doses without compromising target coverage.
Including the IMC lymph nodes in the CTV is debated, due to conflicting results in outcome gain and increased risk of cardiac mortality. However, the results of the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis demonstrate the importance of local treatment on long-term survival, and the fact that IMC lymph node treatment was included in 24 of 25 post-mastectomy RT studies included in the meta-analysis have led to renewed interest in IMC lymph node treatment of breast cancer [Citation1]. Lymphoscintigraphy studies have shown that about one of 25 breast cancer patients have IMC lymph node metastases [Citation11], and that medial tumors are more likely than lateral tumors to drain to the IMC lymph nodes [Citation1,Citation12]. The incidence of lymph node metastases of the internal mammary and medial supraclavicular lymph node chain are reported to be in the range 4–9% in axillary node negative patients and 16–52% for axillary node positive patients [Citation2,Citation13]. Studies on large series of breast cancer patients have shown that patients who had their tumor located in the inner portion of the breast had a significantly worse prognosis than patients with the tumor in the outer quadrant [Citation14,Citation15], which might be due to inadequate treatment of IMC lymph node metastases. Local recurrence in the IMC may be underdiagnosed, and involvement of the IMC lymph nodes might lead to spread of cancer to other regions, such as the pleura and the thoracic cavity [Citation2]. Several authors emphasize the importance of including IMC mapping in breast cancer staging and breast cancer management decision [Citation1,Citation2,Citation14,Citation15], and recommend radiation treatment specifically targeting the IMC lymph nodes if it can be safely administered without significant dose to the heart and the ipsilateral lung.
Loco-regional RT of left-sided breast cancer represents a treatment planning challenge when the IMC lymph nodes are included in the target volume. In this study a mono-isocentric four-field wide tangential technique was applied. The wide tangential fields were necessary to cover the internal mammary nodes, but increased the volume of heart, ipsilateral lung and contralateral breast included in the beam portals. In our study the choice of gantry angle was a compromise between lung and contralateral breast sparing. A deep angle would irradiate much heart and lung, whereas a shallow angle would irradiate much of the contralateral breast. During DIBH, the PTV was moved cranially and the fossa supraclavicular region was squeezed and became shorter. The internal mammary region increased in length (craniocaudally) during DIBH, probably due to the increased intercostal distance during inspiration. The AP-PA fields covering the fossa supraclavicular region were therefore in general shorter in the craniocaudal direction in DIBH plans than in FB plans, while the deep tangential fields covering the IMC region were larger. The ipsilateral lung saving effect due to smaller AP-PA-fields was probably cancelled out by the larger wide tangential beam portals to cover the enlarged IMC region during DIBH. Thus, the cardiopulmonary dose reduction observed during DIBH is mostly caused by the movement of the heart out of the high dose region and the increased lung volume.
The treatment planning was done with focus on similar and good PTV coverage (V95% > 98%) for both breathing techniques. Good PTV coverage is a goal in RT, but not always achievable in clinical practice, where compromising target dose against OAR is a common challenge. Our study shows the cardiopulmonary doses as they would be, with optimal target coverage without compromises.
The FB and DIBH plans in our study showed equal and good PTV coverage as measured by V95% and D98% as well as with HI and CI indices. All plans fulfilled the strict planning criterion of V95% > 98%, and the 95%-isodose was by visual inspection ensured to cover the dorsal part of PTV including the internal mammary nodes with margins. It should be noted that the PB algorithm overestimates the target dose as discussed by Vikström et al. [Citation6]. In clinical practice it is recommended to use a modern calculation algorithm with inhomogeneity corrections that also account for lateral electron scatter, i.e. the analytical anisotropic algorithm (AAA) or the collapsed cone (CC) algorithm. However, as most previous studies used the same algorithm, if stated, the comparison of doses is relevant. Both plans had a CIHealthy tissue larger than 0.6, and can be considered to be conformal according to Lomax and Scheib [Citation16], who defined irradiation to be conformal if the CIHealthy tissue index was ≥0.6. However, the DIBH plans had a slightly decreased normal tissue conformity compared to FB plans, due to a 50 cm3 larger TV95%. The increased TV95% as well as the slightly increased average D2% in the DIBH plans might be due to the increased photon beam transmission caused by decreased lung density during DIBH. The difference in the CI between the FB and DIBH plans is expected to be larger when the AAA or CC algorithm is used.
The DVHs in demonstrate the great benefit of the DIBH technique with respect to cardiopulmonary doses. The volume of heart and lungs irradiated to high-, medium- and low-dose levels were considerably reduced with the DIBH technique. Eleven patients fulfilled the national guidelines with respect to pulmonary doses using the DIBH technique without compromising the PTV coverage, whereas no patients did using the FB technique. Average mean heart dose was halved and only one patient had V25Gy >5% with DIBH. However, all patients benefited from using the DIBH technique with respect to reduced cardiopulmonary doses.
Our study confirms the results of previous studies. Stanzl et al. found in a dose plan study including 11 patients, a similar reduction in mean dose to the entire heart from 4 Gy to 2.5 Gy using DIBH [Citation3]. The lower dose reported in that study might be due to a smaller CTV which only included the breast and the IMC lymph nodes. However, Stranzl et al. did not report anything about the dose coverage to PTV, which is essential when OAR doses are to be compared. Pedersen et al. also found a reduction in cardiopulmonary doses using DIBH for seven patients with left-sided breast tumors where IMC and supraclavicular lymph nodes were included in the PTV [Citation4]. Median heart volume receiving >50% of the prescribed CTV dose was reduced from 8% using FB to 1% using DIBH, and the median ipsilateral relative lung volume receiving >50% of the prescribed dose was reduced from 37% for FB to 31% for DIBH. The reduction in median V25Gy for ipsilateral lung was similar in our study (42.8% for FB vs. 30.4% for DIBH), while the reduction in median V25Gy for the heart was larger (4.5% for FB vs. 0.1% for DIBH). The increased reduction in median V25Gy for the heart might be due to the larger DIBH amplitude in our study. The median V25Gy to ipsilateral lung was not decreased in our study as compared to the study by Pedersen et al., even though the DIBH amplitude was larger. This might be due to better PTV coverage in our study and the use of different gantry angles.
A preliminary analysis of updated EBCTCG data has related mortality from heart disease to estimated cardiac doses in over 30 000 female breast cancer patients followed for up to 20 years. There is clear evidence that the radiation-related increase in mortality is higher in trials with larger mean cardiac RT doses and that the risk of death from heart disease increases by 3% per Gy [Citation17]. Several other studies have also shown that radiation related heart disease can occur following doses below 20 Gy, which emphasize the importance of reducing the mean dose to the heart [Citation17]. Even a mean heart dose of about 4 Gy has been related to development of coronary heart disease [Citation17]. The LAD coronary artery which supplies a significant volume of the heart is particularly vulnerable when the breast or chest wall is treated by tangential fields [Citation18]. This demonstrates the importance of developing techniques that reduce the radiation dose to heart and LAD coronary artery. In our study, the mean dose to the heart was reduced with 50% and mean dose as well as D2% to LAD coronary artery were reduced with 56.4% and 38.5%, respectively, using the DIBH technique.
Pulmonary complications may also be induced by RT for breast cancer patients, and radiation pneumonitis is one of the most common clinical toxicities in these patients [Citation19]. The risk of symptomatic pneumonitis requiring treatment may increase to 4% in patient with regional node irradiation, compared to about 1% when irradiation is confined to the breast [Citation18]. The first results from the EORTC trial 22922/10925 show that the lung toxicity (fibrosis; dyspnoea; pneumonitis; any lung toxicities) at three years in stage I to III breast cancer was significantly increased with internal mammary and medial supraclavicular lymph node irradiation (4.3% vs. 1.3%) [Citation20]. Various Vx of ipsilateral lung i.e. V5Gy, V13Gy, V20Gy, V25Gy and V30Gy, are associated with radiation pneumonitis risk, which suggests that there is no sharp dose threshold below which there is no risk [Citation19]. In our study a reduction in DVH parameters for total as well as ipsilateral lung was observed with DIBH. Considering the national guidelines, a cut-off value of ipsilateral lung V20Gy = 35%, all 17 patients exceeded this limit with FB compared to only six patients using DIBH. Average mean total lung dose was reduced from 10.3 Gy with FB to 8.1 Gy with DIBH, corresponding to a risk of radiation pneumonitis of 7.1% and 5.5%, respectively [Citation19]. The risk is considered low in both cases. The average MLD was increased in DIBH plans as compared to FB plans, suggesting an increased risk for pulmonary complications. However, MLD may not be used as an indicator of pulmonary complications using the DIBH technique, since the relative irradiated lung volume during DIBH is much smaller than during FB.
Mean dose to the contralateral breast increased slightly (0.5 Gy) with DIBH. This result is similar to the one seen by Stanzl et al., who found a non-significant increase in mean dose to contralateral breast from 1.2 Gy with FB to 1.4 Gy with DIBH [Citation3]. The increase is probably of no clinical relevance. In a recent study using the same dataset as in the present study where the treatment was planned for left breast only, no significant difference was found in normal tissue complication probability (NTCP) for contralateral breast using either the DIBH or the FB technique [Citation21]. In this study the AAA was used for dose calculation.
The DIBH technique led to a considerable reduction in both mean dose and average dose maximum (D2%) to the heart. This is an advantage over IMRT, VMAT and tomotherapy, which in several studies have been shown only to reduce the maximum dose, while increasing the mean dose. Goddu et al. and Fogliata et al. found an increase in mean heart dose with tomotherapy for left-sided breast cancer with lymph nodes in the axilla, supraclavicular region and internal mammary region included in the target volume [Citation22,Citation23]. Goddu et al. found a mean heart dose of 12.2 ± 1.8 Gy, and Fogliate et al. found a mean heart dose in the range 8.7–21.1 Gy. Lohr et al. found a 24% increase in mean dose to the heart (from 6.85 Gy to 8.52 Gy) using IMRT of left-sided breast cancer (no lymph nodes included in the target) compared to conventional tangential fields [Citation24]. The increase is primarily caused by the increase in the low-dose volume. In our study mean heart dose was 3.1 ± 1.9 Gy using the DIBH technique. Remouchamps et al. found that radiation during moderate DIBH (using the ABC system from Elekta AB, Sweden) played a larger role in improving the quality of loco-regional breast irradiation including the IMC lymph nodes, than IMRT alone [Citation5]. They found that mean heart V30Gy was reduced from 16.3% with IMRT alone to 3.1% with IMRT in combination with DIBH, thus reducing the absolute mean heart NTCP by 5%. In our study mean heart V25Gy was reduced from 6.7% using FB to 1.2% using DIBH, which might indicate that DIBH alone is recommended for breast irradiation.
In our experience, respiratory gating with the DIBH technique and audiovisual guidance for breast cancer patients is a relatively simple technique to implement in clinical practice [Citation6]. All patients referred for left-sided loco-regional RT are treated with respiratory gating, regardless of age. Patients who receive tangential treatment to the breast only are offered gated RT if they are 60 years or younger. These choices are based on the fact that loco-regional irradiation often results in higher radiation exposure to both the heart and the ipsilateral lung as compared to tangential irradiation only. We only acquired one CT-scan during DIBH. The degree of patient compliance is high. About 1% of the patients have not been able to follow the audio-visual instructions that are necessary for gated RT. This has either been very old and frail patients, patients with a psychiatric disease or mental retardation. The total treatment time per patient at the accelerator is approximately the same as without respiratory gating, and the scan time at the CT is also equal to a CT-scan during FB. The patients need about 20 min to rehearse the DIBH technique. However, the planning procedure itself is usually easier to perform as compared to a non-gated procedure, thus in summary no extra time needs to be calculated for a DIBH plan. The dosimetry is, apart from the effect of decreased lung density, equivalent to conventional RT.
This study shows that respiratory gating during DIBH is a suitable technique for loco-regional breast irradiation even when IMC lymph nodes are included in the PTV. For all patients the cardiopulmonary doses are considerably decreased for all dose levels without compromising the dose coverage to PTV, which is an advantage over IMRT, VMAT and tomotherapy.
Acknowledgements
We thank the Norwegian Cancer Society for the financial support given towards this study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: Diagnosis and implications for patient management – a systematic review. J Clin Oncol 2008;26:4981–9.
- Veronesi U, Arnone P, Veronesi P, Galimberti V, Luini A, Rotmensz N, . The value of radiotherapy on metastatic internal mammary nodes in breast cancer. Results on a large series. Ann Oncol 2008;19:1553–60.
- Stranzl H, Zurl B, Langsenlehner T, Kapp KS. Wide tangential fields including the internal mammary lymph nodes in patients with left-sided breast cancer. Influence of respiratory-controlled radiotherapy (4D-CT) on cardiac exposure. Strahlenther Onkol 2009;185:155–60.
- Pedersen AN, Korreman S, Nystrom H, Specht L. Breathing adapted radiotherapy of breast cancer: Reduction of cardiac and pulmonary doses using voluntary inspiration breath-hold. Radiother Oncol 2004;72:53–60.
- Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys 2003;55:392–406.
- Vikstrom J, Hjelstuen MH, Mjaaland I, Dybvik KI. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol 2011;50:42–50.
- Storchi PR, van Battum LJ, Woudstra E. Calculation of a pencil beam kernel from measured photon beam data. Phys Med Biol 1999;44:2917–28.
- Webb S, Fox RA. Verification by Monte Carlo methods of a power law tissue-air ratio algorithm for inhomogeneity corrections in photon beam dose calculations. Phys Med Biol 1980;25:225–40.
- ICRU Report 83. International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting intensity-modulated photon-beam therapy (IMRT). J ICRU 2010;10.
- Feuvret L, Noel G, Mazeron JJ, Bey P. Conformity index: A review. Int J Radiat Oncol Biol Phys 2006;64:333–42.
- Heuts EM, van der Ent FW, von Meyenfeldt MF, Voogd AC. Internal mammary lymph drainage and sentinel node biopsy in breast cancer – A study on 1008 patients. Eur J Surg Oncol 2009;35:252–7.
- Estourgie SH, Nieweg OE, Olmos RA, Rutgers EJ, Kroon BB. Lymphatic drainage patterns from the breast. Ann Surg 2004;239:232–7.
- Freedman GM, Fowble BL, Nicolaou N, Sigurdson ER, Torosian MH, Boraas MC, . Should internal mammary lymph nodes in breast cancer be a target for the radiation oncologist? Int J Radiat Oncol Biol Phys 2000;46:805–14.
- Colleoni M, Zahrieh D, Gelber RD, Holmberg SB, Mattsson JE, Rudenstam CM, . Site of primary tumor has a prognostic role in operable breast cancer: The international breast cancer study group experience. J Clin Oncol 2005;23:1390–400.
- Sarp S, Fioretta G, Verkooijen HM, Vlastos G, Rapiti E, Schubert H, . Tumor location of the lower-inner quadrant is associated with an impaired survival for women with early-stage breast cancer. Ann Surg Oncol 2007;14:1031–9.
- Lomax NJ, Scheib SG. Quantifying the degree of conformity in radiosurgery treatment planning. Int J Radiat Oncol Biol Phys 2003;55:1409–19.
- Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, . Radiation-related heart disease: Current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010;76:656–65.
- Kunkler IH. Radiotherapy of the regional lymph nodes: Shooting at the sheriff? Breast 2009;18(Suppl 3):S112–20.
- Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, . Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76(3 Suppl):S70–6.
- Matzinger O, Heimsoth I, Poortmans P, Collette L, Struikmans H, Van Den BW, . Toxicity at three years with and without irradiation of the internal mammary and medial supraclavicular lymph node chain in stage I to III breast cancer (EORTC trial 22922/10925). Acta Oncol 2010;49:24–34.
- Johansen S, Vikstrom J, Hjelstuen MH, Mjaaland I, Dybvik KI, Olsen DR. Dose evaluation and risk estimation for secondary cancer in contralateral breast and a study of correlation between thorax shape and dose to organs at risk following tangentially breast irradiation during deep inspiration breath-hold and free breathing. Acta Oncol 2011;50:563–8.
- Fogliata A, Nicolini G, Alber M, Asell M, Dobler B, El-Haddad M, . IMRT for breast. A planning study. Radiother Oncol 2005;76:300–10.
- Goddu SM, Chaudhari S, Mamalui-Hunter M, Pechenaya OL, Pratt D, Mutic S, . Helical tomotherapy planning for left-sided breast cancer patients with positive lymph nodes: Comparison to conventional multiport breast technique. Int J Radiat Oncol Biol Phys 2009;73:1243–51.
- Lohr F, El-Haddad M, Dobler B, Grau R, Wertz HJ, Kraus-Tiefenbacher U, . Potential effect of robust and simple IMRT approach for left-sided breast cancer on cardiac mortality. Int J Radiat Oncol Biol Phys 2009;74: 73–80.