Dear Editor,
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the progressive accumulation of phenotypically mature malignant lymphocytes [Citation1]. B-CLL therapy remains unsatisfactory due to repeated resurgences of the chemoresistant disease. Chemoresistance remains a major therapeutic challenge in B-CLL. The precise mechanism underlying chemoresistance in B-CLL is not clear, but one of the main contributors is thought to be a dysregulation of apoptosis, and an overexpression of antiapoptotic molecules, such as cytokines [Citation2].
B-CLL cells commonly express several transporter proteins involved in multidrug resistance including P-glycoprotein (P-gp, also known as MDR1 and ABCB1), multidrug resistance-associated protein-1 (MRP1, ABCC1) and lung resistance protein (LRP) [Citation3]. A relatively high level of P-gp expression, the classical mechanism of multidrug resistance, appears to be an intrinsic feature of B-CLL tumor cells [Citation3].
A number of single nucleotide polymorphisms (SNPs) were identified in the MDR1 gene locus, including a synonymous or silent C to T transition at position 3435 in exon 26 that was associated with altered expression and activity of P-gp [Citation4]. Jamroziak et al. showed that a silent polymorphism at position 3435 of the MDR1 gene is associated with a predisposition to B-CLL and determines P-gp activity in chemotherapy-naive B-CLL tumor cells [Citation5].
The aim of our study was to evaluate plasma levels of Interleukin-22, IL-23 and IL-10 in patients with B-CLL with different MDR-1 gene polymorphisms. We evaluated two MDR-1 gene polymorphisms: G2677T polymorphism in exon 21 and C3435T polymorphism in exon 26, to see if polymorphisms influence the plasma concentration of the cytokines studied.
The study was conducted in accordance with the Declaration of Helsinki, and it has been approved by the local ethics committee. Fully informed consent was obtained in writing from each patient.
The study population included 27 patients with B-CLL. Subjects were newly diagnosed patients (15 female and 12 male) with a median age of 73 ± 7 years.
The diagnosis of B-CLL was established according to the National Cancer Institute- (NCI-) Sponsored Working Group diagnostic criteria for CLL. According to Rai classification, 10 patients were in stage 0, whereas six were in stage I, four in stage II, and the remaining seven were in stage III–IV.
IL-22, IL-23 and IL-10 plasma concentrations were measured by a quantitative enzyme immunoassay technique. The assays were performed by using commercially available kits (R&D Systems Europe, Abingdon, UK); a microplate reader (BioRad Laboratories, Model 550, Milan) capable of measuring absorbance at 450 nm (correction wavelength set at 540 nm) was used to measure the intensity of color developed in each well. All samples were analyzed in duplicate.
For MDR-1 genotyping, the genomic DNA was extracted from patients’ peripheral blood using standard procedures. Two MDR-1 gene polymorphisms were detected based on polymerase chain reaction (PCR), using primers amplifying a short fragment of DNA containing the polymorphic sites. The PCR primers for G2677T polymorphism in exon 21 were 5′-TGC AGG CTA TAG GTT CCA GG-3′ and 5′-TTT AGT TTG ACT CAC CTT CCC G-3′, generating a 224-bp fragment, while those for the C3435T polymorphism in exon 26 were 5′-GCT GCT TGA TGG CAA AGA AA-3′ and 5′-ATT AGG CAG TGA CTC GAT GAT GA-3′, generating a 208-bp fragment. The PCRs were performed in a 40-μl reaction volume containing 100 ng of genomic DNA, 10 pmol of each primer, 0.2 mM of each deoxynucleotide triphosphate, 1X PCR buffer [50 mM KCl and 10 mM Tris-HCl (ph 8,3)], 1.5 mM MgCl2 and 1 unit of Taq polymerase. The PCR program for exon 21 consisted of 35 cycles at 94°C for 30 s, 58°C for 30 s, 72°C for 30 s, and a final elongation step at 72°C for 10 min. The PCR program for exon 26 consisted of 35 cycles at 94°C for 30 s, 54°C for 30 s, 72°C for 30 s, and a final elongation step at 72°C for 10 min. The PCR products were checked on a 1.5% agarose gel, photographed using Polaroid film, and subjected to a RFLP analysis.
To distinguish the SNPs, the restriction enzyme BanI (New England BioLabs) was used for G2677T and DpnII (New England BioLabs) for C3435T. Each 20 μL of PCR products was digested overnight with 5 units of BanI (G2677T in exon 21) and DpnII (C3435T in exon 26) at 37°C. The digestion products were separated on a 4% agarose gel.
We used this method for MDR1 evaluation (MDR1 gene RFLPs) because of its simplicity, specificity, reproducibility and its cost, while several authors have reported the same method in their works [Citation6].
The statistical analysis was performed with SPSS for Windows (version 17.0). Data were presented as mean ± standard deviation (SD). Differences between data series were analyzed by the Mann-Whitney test. Statistical significance was set at p < 0.05.
No statistically significant differences were found between plasma levels of IL-10 or IL-23 of B-CLL patients with respect to the considered polymorphisms.
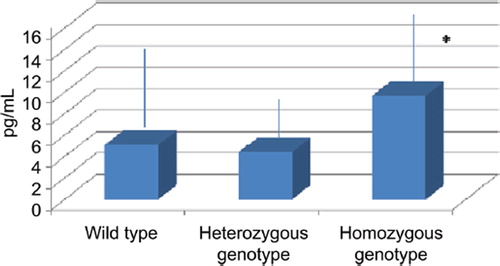
There was a significant difference between the plasma levels of IL-22 in patients with the mutant heterozygous 2677 GT genotype and those measured in the mutant homozygous 2677 TT genotype (4.40 ± 2.67 pg/ml vs. 9.68 ± 6.13; p < 0.049) (). No difference was found between IL-22 plasma levels in wild type and heterozygous patients or between wild type patients and homozygous subjects.
Figure 1. Increase of IL-22 plasma concentration in B-CLL patients presenting T2677T MDR-1 gene polymorphism with respect to G2677T; *p < 0.04.
As regards to the C3435T MDR1 polymorphism, we were not able to identify any significant correlation with IL-22 plasma levels.
The etiology of B-CLL is poorly understood, though both genetic and environmental factors appear to contribute to leukemogenesis [Citation7]. In regard to genetic predisposition, an autosomal dominant mode of transmission was described in some cases with a rare familial subset of B-CLL [Citation8].
In a previous work we pointed out that a higher T allele frequency is observed in patients with B-CLL when compared with controls [Citation9]. Moreover, we found that IL-22 plasma levels were significantly increased in B-CLL patients with respect to controls (data unpublished), and this increase was more pronounced in patients with high levels of CD38 expression, a well known marker of poor prognosis.
P-Glycoprotein is a cell membrane-associated protein that transports a variety of exogenous (including drugs) and endogenous substances. P-Glycoprotein may also be involved in transmembrane transport of some endogenous proteins; thus, it may have physiological function in cytokine transport. Pawlik et al. suggested an association between C3435T and G2677T MDR1 polymorphisms and transmembrane transport of some cytokines such as INF-gamma, IL-2, IL-4 and TNF-alpha [Citation10], while in ovarian cancer cells Wang et al. demonstrated that the chemoresistance caused by IL-6 is associated with increased expression of multidrug resistance-related genes [Citation11].
In our study we demonstrated an increase of IL-22 plasma concentration in B-CLL patients presenting T2677T multidrug resistance gene polymorphism.
Interleukin (IL)-22 is produced by Th17, Th22, Th1 cells, classical and non-classical (NK-22) NK cells, NKT cells, and lymphoid tissue inducer cells. Only activated T cells and activated NK cells express IL-22, whereas resting or activated B cells, monocytes, monocyte-derived macrophages and immature and mature dendritic cells were not able to produce this cytokine [Citation12,Citation13].
An IL-22 increase could justify the B-CLL chemoresistance in patient with 2677 TT genotype.
One of the main contributors to both B-CLL chemoresistance and pathogenesis in fact is thought to be activation of NF-κB and STAT3 [Citation14], and Bard et al. described the existence of an autocrine stimulatory loop involving interleukin-22 that contributes to STAT3 activation and tumorigenicity of anaplastic large cell lymphoma (ALK(+)ALCL) [Citation15]. Moreover, Dumontier et al. have demonstrated that aside from the classical STAT recruitment via its four potential STAT binding sites, IL-22R1 is pre-associated with the major IL-22 signaling molecule STAT3 as a result of a tyrosine-independent new mode of STAT3 recruitment via interaction of its coiled-coil-domain with the Cterminal end of IL-22R1 [Citation16].
Finally, NFKB pathway may also have a role in suppressing the apoptosis in B-CLL cells [Citation17], and Gelebard et al. analyzed the gene promoter of IL-22RA1, and they found multiple binding sites for NF-κB [Citation18].
In conclusion, our data provides a rationale to explore if pharmacological inhibition of IL-22 could result in a substantial reduction of B-CLL chemoresistence in selected patients with specific multidrug resistance gene polymorphisms.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med 2005; 352:804–15.
- Allen JC, Talab F, Zuzel M, Lin K, Slupsky JR. c-Abl regulates Mcl-1 gene expression in chronic lymphocytic leukemia cells. Blood 2011;117:2414–22.
- Consoli U, Santonocito A, Stagno F, Fiumara P, PriviteraA, Parisi G, . Multidrug resistance mechanisms in chronic lymphocytic leukemia. Br J Haematol 2002; 116:774–80.
- Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, . Functional polymorphisms of the human multidrug resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA 2000; 97:3473–8.
- Jamroziak K, Balcerczak E, Smolewski P, Robey RW, Cebula B, Panczyk M, . MDR1 (ABCB1) gene polymorphism C3435T is associated with P-glycoprotein activity in B-cell chronic lymphocytic leukemia. Pharmacological Report 2006;58:720–8.
- Goreva OB, Grishanova AY, Mukhin OV, Domnikova NP, Lyakhovich VV. Possible prediction of the efficiency of chemotherapy in patients with lymphoproliferative diseases based on MDR1 gene G2677T and C3435T polymorphisms. Bull Exp Biol Med 2003;136:183–5.
- Houlston RS, Catovsky D, Yuille MR Genetic susceptibility to chronic lymphocytic leukemia. Leukemia 2002;16:1008–14.
- Lynch HT, Weisenburger DD, Quinn-Laquer B, Watson P, Lynch JF, Sanger WG.Hereditary chronic lymphocytic leukemia: An extended family study and literature review. Am J Med Genet 2002;115:113–7.
- Penna G, Allegra A, Alonci A, Aguennouz M, Garufi A, Cannavò A, . MDR-1 polymorphisms (G2677T and C3435T) in B-chronic lymphocytic leukemia: An impact on susceptibility and prognosis. Med Oncol Epub 2010 May 22.
- Pawlik A, Baskiewicz-Masiuk M, Machalinski B, Kurzawski M, Gawronska-Szklarz B. Involvement of C3435T and G2677T multidrug resistance gene polymorphisms in release of cytokines from peripheral blood mononuclear cells treated with methotrexate and dexamethasone. Eur J Pharmacol 2005; 528:27–36.
- Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ, . Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells. Cancer Lett 2010;295:110–23.
- Dumoutier L, Van Roost E, Colau D. Human interleukin-10-related T cell derived inducible factor: Molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc Natl Acad Sci USA 2000;97:10144–9.
- Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, Stinson J, . Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor related proteins CRF2-4 and IL-22R. J Biol Chem 2000;275:31335–9.
- Hsu HS, Huang PI, Chang YL, Tzao C, Chen YW, Shih HC, . Cucurbitacin I inhibits tumorigenic ability and enhances radiochemosensitivity in nonsmall cell lung cancer-derived CD133-positive cells. Cancer 2011;117:2970–85;
- Bard JD, Gelebart P, Anand M, Amin HM, Lai R. Aberrant expression of IL-22 receptor 1 and autocrine IL-22 stimulation contribute to tumorigenicity in ALK+ anaplastic large cell lymphoma. Leukemia 2008;22:1595–603.
- Dumoutier L, de Meester C, Tavernier J, Renauld JC.New activation modus of STAT3: A tyrosine-less region of the interleukin-22 receptor recruits STAT3 by interacting with its coiled-coil domain. J Biol Chem 2009;284:26377–84.
- Lyng H, Landsverk KS, Kristiansen E, DeAngelisPM, Ree AH, Myklebost O, .Response of malignant B lymphocytes to ionizing radiation: Gene expression and genotype. Int J Cancer2005;115:935–42.
- Gelebart P, Zak Z, Dien-Bard J, Anand M, Lai R. Interleukin 22 signaling promotes cell growth in mantle cell lymphoma. Transl Oncol 2011;4:9–19.