Abstract
Background. Chemoradiation improves survival for patients with locally advanced non-small cell lung cancer (NSCLC), but clinical outcomes beyond five years are rarely reported. The aim of the present study was to identify the long-term results of a phase II study of docetaxel and cisplatin with concurrent thoracic radiation. Methods. We previously reported short-term outcomes from the phase II study, which enrolled 42 patients (aged ≤ 75 years) with unresectable stage III NSCLC. We continued to follow these patients for long-term clinical outcomes. Results. At a median follow-up for all patients of 6.3 years (range: 5.2–7.1 years), the median survival time was 2.1 years and the actual five-year survival rate was 31%. Among 14 patients who were progression-free longer than two years, three patients died due to bacterial or fungal pneumonia and one died due to gall bladder cancer. Conclusions. Thirty-one percent of locally advanced patients having NSCLC treated with docetaxel and cisplatin and concurrent thoracic radiation survived beyond five years. Progression-free patients might be cautiously followed up taking precautions against emerging pneumonia.
Concurrent cisplatin-based chemoradiation has been the standard treatment for patients having locally advanced non-small cell lung cancer (LA-NSCLC), with good performance status and adequate organ function [Citation1]. We reported a phase I/II study of docetaxel and cisplatin (DP) chemotherapy with concurrent thoracic radiation (TRT) for LA-NSCLC, in which 54% of the patients had a two-year survival with acceptable acute toxicities [Citation2]. Subsequently, we conducted a phase III study comparing the DP regimen with the mitomycin, vindesine, and cisplatin (MVP) regimen when TRT was concurrently performed for LA-NSCLC [Citation3]. No significant efficacy difference in the survival time at two years between the treatment arms based on primary analysis by the stratified log-rank test was observed; however, a secondary result based on the modified log-rank test appeared to indicate that DP has better efficacy for the early follow-up period.
Although some long-term follow-up data regarding chemotherapy with TRT for LA-NSCLC were reported [Citation4–9], those regarding the DP regimen with TRT remain unknown. In the present study, we report long-term results of the phase II study.
Patients and methods
The methods used have been previously described [Citation2]. Briefly, patients (≤75 years old) with unresectable IIIA or IIIB NSCLC and Eastern Cooperative Oncology Group performance status (PS) 0 or 1 were eligible. Docetaxel (40 mg/m2) was administered intravenously, followed by cisplatin (40 mg/m2), on Days 1, 8, 29, and 36. Concurrent radiotherapy began on Day 1 of chemotherapy and was administered in daily radiation doses of 2 Gy for five consecutive days every week, for a total dose of 60 Gy using a linear accelerator (6–10 MeV). A curative radiation field was constructed using a plain chest radiograph and a contrast-enhanced computed tomography (CT)-scan. The initial dose (∼40 Gy) was administered to the primary tumor, the ipsilateral hilum with a 2-cm margin, and the involved mediastinal lymph nodes with a 1-cm margin. Prophylactic radiation fields were not planned, except for subcarinal lymph nodes. Subsequently, an additional 20 Gy dose was administered to the boost volume, including the sites of primary tumor and hilar/mediatinal lymph nodes according to the tumor and lymph nodes shrinkage determined by contrast-enhanced CT-scan on Day 29 or later. The chemotherapy and radiation doses and schedules were modified in cases of toxicity, as described previously. Written informed consent was obtained from all patients. The survival time was defined as the period from initiation of treatment to death or last follow-up evaluation, and progression-free survival (PFS) was defined as the period from initiation of treatment to progression or death due to any causes. The overall survival (OS) and PFS curves were calculated using the Kaplan-Meier method.
The dose intensities of cisplatin, docetaxel and radiotherapy were calculated as the ratios of administered drug (mg/m2/week) or actual radiation dose (Gy/week) to projected drug dose (mg/m2/week) or projected radiation dose (Gy/week), respectively. The values represented mean ± standard deviation. P-value less than 0.05 by Student's t-test was considered statistically significant. All statistical analyses were performed using the SPSS software package (version 11.0J; SPSS Inc., Chicago, IL, USA).
Results
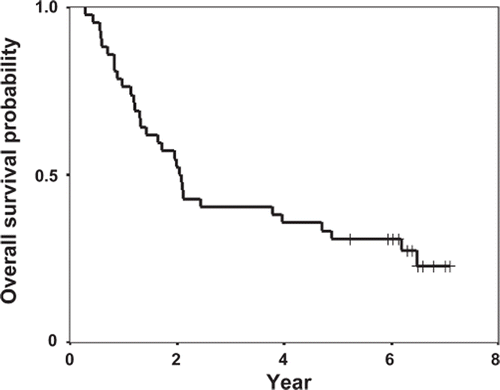
Between September 1998 and December 1999, the Okayama Lung Cancer Study Group conducted the present phase II study [Citation2]. Patient characteristics are shown in . The median follow-up for all patients was 6.3 years (range: 5.2–7.1 years). Five patients received salvage chemotherapy after relapse. Post progression therapy including chest radiation or surgery was not conducted. shows the OS curve. The median survival time was 2.1 years (95% confidence interval [CI]: 0.82–2.5 years), and the actual number of five-year survivors was 13 (31%). Two of the 13 survivors died (one due to recurrence and one due to gall bladder cancer) and one was lost to follow-up after five years.
Table I. Patient characteristics.
Figure 1. Overall survival curve from the initiation of docetaxel, cisplatin, and concurrent radiation.
The median PFS time was 1.16 years (95% CI: 0.35–1.96 years; ). Among 14 patients who were progression-free longer than two years, three patients died due to bacterial or fungal pneumonia and one died due to gall bladder cancer (). In all the three patients who died due to pneumonia, the infiltrative shadow debuted from outside the irradiated volume. In 10 patients without recurrence, all had good PS and the Hugh-Jones classification scores were 0 (eight patients) or 3 (two patients). The two patients with scores of 3 were aged 74 and 78 years. The grades of late-radiation pneumonitis were all 1 and late-radiation esophagitis was not observed.
Figure 2. Progression-free survival curve from the initiation of docetaxel, cisplatin, and concurrent radiation.
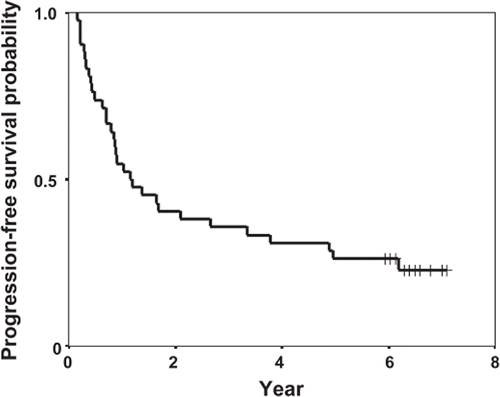
Table II. Characteristics of patients who were progression-free more than two years.
As shown in , the dose intensity of cisplatin (88.9%) in long survivors without progression, who were described in , was higher than that (85.6%) in others (p = 0.022). However, there were no differences in dose intensities of docetaxel and radiation between the two groups.
Table III. Dose intensities for cisplatin, docetaxel and radiotherapy.
Discussion
The DP-TRT regimen in the present phase II study was effective with a median survival time of 2.1 years and an actual five-year survival rate of 31%. Among 14 patients who were progression-free longer than two years, three patients died due to pneumonia and one died due to a second primary cancer.
The five-year survival rate of the patients having LA-NSCLC treated with concurrent chemoradiation in a meta-analysis was 8.2% [Citation8]. The five-year survival rate of the patients enrolled in six Japanese phase II studies, including five concurrent chemoradiation and one alternating chemoradiation, was 14.4% [Citation9]. The prognosis of those patients remains poor. The patients registered in our phase II study might be selected because of excellent efficacy. Disappointedly, the expected five-year OS and PFS rate of the DP-TRT arm in the phase III study were 23.5% and 16.3%, respectively, although this was not a long-term result [Citation3]. All the long-term survivors in the present study maintained good PS. The two patients with Hugh-Jones classification scores of 3 might have had the scores due to age rather than long-term toxicity. Thus, the survivors would benefit from this regimen without severe late toxicities, such as radiation fibrosis or esophagitis. The long-term outcomes in the phase III study to ascertain the results of this phase II study will be reported in the near future [Citation3].
The causes of death other than NSCLC were pneumonia and second primary cancer. We reported that second primary cancer occurred and long-term survivors required vigilant follow-up to detect second primary cancer at the earliest possible stage [Citation10]. Notably, three patients died of bacterial or fungal pneumonia. Although the patients did not have late lung toxicities, radiation might have caused damage to the in-field normal lungs. These patients may likely have been affected by respiratory infection and may not have recovered from it easily. Thus, we might need to treat the patients with pneumonia intensively.
This trial has a limitation concerning radiation therapy planning. The planning target volume (PTV) was not calculated in all the institutions because CT simulation was not mandatory for this trial. Approximately two-thirds of the institutes used CT simulation to construct the radiation field at the beginning of this trial. In 23 patients treated at these institutions, normal tissues volumes were as follows: normal lung volume ≤25%, 30 Gy or less, spinal cord, 45 Gy or less. Unfortunately, dose volume histograms data were not collected in the case report forms. CT-based simulation has been widely adopted for the treatment of LA-NSCLC and is associated with higher survival among patients receiving TRT [Citation11].
Recently, three new regimens of cisplatin and docetaxel with TRT were reported. Two cycles of docetaxel 75 mg/m2 on Day 1 and cisplatin 40 mg/m2 on Days 1 and 2 were administered, either preceding or following concurrent chemoradiation; 66 Gy was delivered using involved fields concurrent with weekly docetaxel 20 mg/m2 and cisplatin 20 mg/m2 [Citation12]. At a median follow-up of 14.3 months, the median OS of all eligible patients was 28.0 months and the median PFS was 9.5 months. Kocak et al. reported that docetaxel 75 mg/m2 on Day 1 every three weeks and cisplatin 75 mg/m2 on Day 1 every three weeks followed by chemoradiation (docetaxel 30 mg/m2 every week and cisplatin 20 mg/m2 every week with 66 Gy of radiation therapy) was excellent: the median OS was 29.9 months and median PFS was 15 months at a median follow-up of 15.7 months [Citation13]. Additionally, induction chemotherapy consisting of 75 mg/m2 docetaxel and 75 mg/m2 cisplatin on Day 1 and 150 mg/m2 of recombinant human granulocyte colony-stimulating factor on Days 2–10 was reported. In the regimen, concurrent chemoradiation was started three to six weeks later with twice-weekly docetaxel at 10–12 mg/m2 and daily delayed radiation in 1.8-Gy fractions to 64.5 Gy for gross disease [Citation14]. The pilot clinical study resulted in the median OS of 32.6 months with three- and five-year survival rates of 50% and 19%, respectively. Although a longer follow-up of the studies and a randomized phase III trial are required to confirm the safety and efficacy of the regimen, chemotherapy consisting of cisplatin and docetaxel with TRT for LA-NSCLC seemed very effective.
Machtay et al. recently reported that higher radiotherapy dose intensity was associated with improved local-regional control and survival in the setting of chemoradiotherapy although they did not refer the dose intensity of chemotherapeutic drugs [Citation15]. Our study suggested that the dose intensity of cisplatin in the DP-TRT regimen might play a role in long survival.
In conclusion, substantial long-term survivors after the DP-TRT regimen for LA-NSCLC was demonstrated in the phase II study. The occurrence of pneumonia and secondary primary cancer in the long-term survivors should be a concern in the follow-up.
Acknowledgments
We thank the patients who consented to this trial, their families, and members of the Okayama Lung Cancer Study Group for their collaborations.
Declaration of interest: Drs. Takigawa and Kiura were paid an honorarium for lecturing from Sanofi-Aventis, Japan. The other authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Blackstock AW, Govindan R. Definitive chemoradiation for the treatment of locally advanced non small-cell lung cancer. J Clin Oncol 2007;25:4146–52.
- Kiura K, Ueoka H, Segawa Y, Tabata M, Kamei H, Takigawa N, . Phase I/II study of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small-cell lung cancer. Br J Cancer 2003;89:795–802.
- Segawa Y, Kiura K, Takigawa N, Kamei H, Harita S, Hiraki S, . Phase III trial comparing docetaxel and cisplatin combination chemotherapy with mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiotherapy in locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol 2010;28:3299–306.
- Fournel P, Robinet G, Thomas P, Souquet PJ, Lena H, Vergnenegre A, . Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol 2005;23:5910–7.
- Kubota K, Tamura T, Fukuoka M, Furuse K, Ikegami H, Ariyoshi Y, . Phase II study of concurrent chemotherapy and radiotherapy for unresectable stage III non-small-cell lung cancer: Long-term follow-up results. Japan Clinical Oncology Group Protocol 8902. Ann Oncol 2000;11:445–50.
- Kim DW, Shyr Y, Shaktour B, Akerley W, Johnson DH, Choy H. Long term follow up and analysis of long term survivors in patients treated with paclitaxel-based concurrent chemo/radiation therapy for locally advanced non-small cell lung cancer. Lung Cancer 2005;50:235–45.
- Keene KS, Harman EM, Knauf DG, McCarley D, Zlotecki RA. Five-year results of a phase II trial of hyperfractionated radiotherapy and concurrent daily cisplatin chemotherapy for stage III non-small-cell lung cancer. Am J Clin Oncol 2005;28:217–22.
- Auperin A, Le Pechoux C, Pignon JP, Koning C, Jeremic B, Clamon G, . Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): A meta-analysis of individual data from 1764 patients. Ann Oncol 2006;17:473–83.
- Ohe Y, Ishizuka N, Tamura T, Sekine I, Nishiwaki Y, Saijo N. Long-term follow-up of patients with unresectable locally advanced non-small cell lung cancer treated with chemoradiotherapy: A retrospective analysis of the data from the Japan Clinical Oncology Group trials (JCOG0003A). Cancer Sci 2003;94:729–34.
- Takigawa N, Kiura K, Segawa Y, Watanabe Y, Kamei H, Moritaka T, . Second primary cancer in survivors following concurrent chemoradiation for locally advanced non-small-cell lung cancer. Br J Cancer 2006;95:1142–4.
- Chen AB, Neville BA, Sher DJ, Chen K, Schrag D. Survival outcomes after radiation therapy for stage III non-small-cell lung cancer after adoption of computed tomography-based simulation. J Clin Oncol 2011;29:2305–11.
- Senan S, Cardenal F, Vansteenkiste J, Stigt J, Akyol F, De Neve W, . A randomized phase II study comparing induction or consolidation chemotherapy with cisplatin-docetaxel, plus radical concurrent chemoradiotherapy with cisplatin-docetaxel, in patients with unresectable locally advanced non-small-cell lung cancer. Ann Oncol 2011;22:553–8.
- Kocak M, Ozkan A, Mayadagli A, Parlak C, Bilici A, Seker M, . Induction chemotherapy and chemoradiation therapy for inoperable locally advanced non-small-cell lung cancer: A single-institution review of two different regimens. Clin Lung Cancer 2009;10:124–9.
- Chen Y, Pandya KJ, Hyrien O, Keng PC, Smudzin T, Anderson J, . Preclinical and pilot clinical studies of docetaxel chemoradiation for stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2010;80:1358–64.
- Machtay M, Bae K, Movsas B, Paulus R, Gore EM, Komaki R, . Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: An analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys Epub 2010 Oct 25.