To the Editor
Langerhans cell histiocytosis (LCH) is a rare idiopathic disease with diverse clinical manifestations ranging from a single osteolytic lesion to generalized disease. Various treatment regimens have been proposed for the multisystem type, however, with inconsistent outcomes. Herein we are the first to report on a therapy effect of a lenalidomide-based regimen in a patient with repeatedly relapsed aggressive form of multisystem LCH.
Case report
A male, born 1973, was diagnosed with multisystem LCH affecting the lymph nodes, skin and lungs at the age of 35. The initial manifestation resembled a lymphoma with expressed B-symptoms (night sweats, fever, weight loss, fatigue) and generalized lymphadenopathy involving the cervical, periclavicular, axillary, retroperitoneal and inguinal lymph nodes. His other medical history was unremarkable, except for a smoking addiction.
Laboratory results included signs of inflammation (erythrocyte sedimentation rate = 31 mm/h, C-reactive protein = 24 mg/l, leukocyte count = 13.6 × 109 cells/l) and a mild normocytic anemia (hemoglobin = 127 g/l). Renal and hepatic profiles were normal. The diagnosis was determined from lymph node and perianal skin biopsies and by a typical finding on high-resolution computed tomography (CT) imaging, pulmonary involvement was confirmed.
In March 2009, the patient underwent a peripheral blood stem cells mobilization (37.29 × 106 CD34 + cells/kg collected) after stimulation regimen with cyclophosphamide (2 g/m2 intravenous on Day 1) and etoposide (200 mg/m2 intravenous on Days 1–3) followed by filgrastim application (5 μg/kg subcutaneous). First line treatment then consisted of cladribine monotherapy (10 mg subcutaneous on Days 1–5 of a 28-day cycle, six cycles in total) enhanced by cyclophosphamide (300 mg intravenous on Days 1–5, cycles 4–6), methylprednisolone (250 mg intravenous on Days 1–5, cycles 4–6) and radiotherapy of the perianal area, achieving complete disease remission. However, after two months the initial B-symptoms occurred again, with hip bone pains as well. The disease relapse was proved by lymph node biopsy and confirmed on a restaging positron emission tomography (PET)/CT examination which also showed disease dissemination into the hip bones.
Therefore, an aggressive form of the disease with an early relapse was diagnosed. The patient underwent a salvage regimen consisting of four cycles of CHOEP chemotherapy (cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 intravenous on Day 1; etoposide 100 mg/m2 intravenous on Days 1–3, prednisone 100 mg perorally on Days 1–5 of a 28-day cycle). Because of severe neutropenia, we had to reduce the intensity of this regimen, so that only etoposide with prednisone were administered in the third and fourth cycle. This treatment was completed in March 2010 with autologous peripheral blood stem cell transplantation after high-dose BEAM chemotherapy (carmustine 300 mg/m2 intravenous on Day -7, etoposide 200 mg/m2 intravenous on Days -6 to -3, cytarabine 200 mg/m2 intravenous twice a day on Days -6 to -3, melphalan 140 mg/m2 on Day -2).
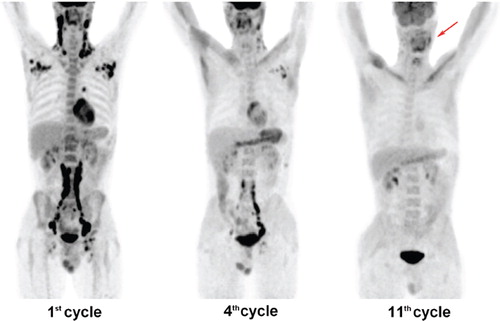
Restaging PET/CT examinations after two cycles of CHOEP chemotherapy, as well as three months after the transplantation showed no pathological tracer uptake. However, the complete remission had only been maintained for five months following the transplantation, when the second disease relapse was diagnosed from a lymph node biopsy in September 2010. PET/CT scanning revealed generalized lymphadenopathy with cervical, supraclavicular, axillary, mediastinal, retroperitoneal and inguinal involvements. By that time, our clinic had enrolled in the Celgene's Compassionate Use Program and received lenalidomide treatment for our patient. Based on our experience, we opted for a dosing scheme used in multiple myeloma, i.e. 25 mg on Days 1–21 in a 28-day cycle. The treatment was enhanced with dexamethasone (40 mg perorally once a week, cycles 1–11) and etoposide (100 mg intravenous on Days 22–24, cycles 6–10), which was stopped in the 11th cycle due to the planned patient's high dose chemotherapy before allogeneic blood stem cell transplantation. Antithrombotic prophylaxis with low-molecular weight heparin was implemented.
Within several doses of lenalidomide, night sweats, fevers and back pain receded gradually, which was followed by a decrease of blood inflammatory markers (C-reactive protein, erythrocyte sedimentation rate). After the second cycle, fatigue diminished and the patient began to feel well. The clinical and laboratory therapy responses were followed by radiologically proved regression of all enlarged lymph nodes. A series of 10 ultrasound and four PET/CT examinations were performed at two to four-week intervals and three-month intervals, respectively. The acquired data, including a focus on two selected lymph nodes, are summarized in showing that a major treatment response took place in the first month of the lenalidomide-based regimen. Moreover, we observed a significant increase in the immunoregulatory index (CD4 + /CD8 + ratio) after eight cycles of lenalidomide treatment (0.156 in September 2010, 0.462 in May 2011). In addition, plasma levels of free circulating interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) were assessed using ELISA kits before and throughout the treatment but these were below detection limits (limits of detection for IL-1β and TNF-α were 0.7 pg/ml and 1.65 pg/ml, respectively).
Table I. Paraclinical findings during the lenalidomide-based treatment.
A restaging PET/CT examination during the fourth cycle showed reduction in the size of the affected lymph nodes and their glucose uptake with physiological findings in the right axilla, right lung hilus and right inguina. Finally, pathological glucose hypermetabolism was detected only in one left cervical lymph node on a PET/CT scan at the end of the 11th cycle (). Though the treatment response was slower than in previous regimens, nearly complete remission has been achieved. Further therapeutic approach in this patient will be targeted to fully eradicate the disease by means of allogeneic blood stem cell transplantation. The overall tolerance of the lenalidomide-based regimen was excellent with no myelotoxic or thromboembolic side effects. Before and after the treatment, leukocyte counts were 12.5 × 109 cells/l and 10.5 × 109 cells/l, hemoglobin levels were 122 g/l and 128 g/l, and thrombocyte counts were 539 × 109 cells/l and 160 × 109 cells/l, respectively.
Discussion
LCH is an uncommon entity characterized by dysregulated proliferation and accumulation of pathological Langerhans cells in various tissues and organs [Citation1]. Although it may occur at all ages, it mostly arises in children aged 1–3 years [Citation2]. In adults the incidence is estimated to be 1–2 cases per million per year [Citation3]. LCH has a broad spectrum of clinical presentations ranging from an indolent single osteolytic lesion to generalized disease with poor prognosis. Any part of the body may be affected, though most commonly involved are the skeletal system, skin and lungs [Citation4,Citation5]. The diagnosis is based on histological and immunophenotypic examinations of lesional cells which typically exhibit positive staining with proteins S-100, CD1a and langerin. According to the Histiocyte Society Evaluation and Treatment Guidelines, vinblastine, prednisone and 6-mercaptopurine are indicated in the first line treatment of multisystem disease, while cladribine (2-chlorodeoxyadenosine), cytarabine and stem cell transplantation are proposed in the second line therapy thereof [Citation6]. However, to our knowledge, there are no recommendations on how to proceed in case of a refractory disease.
Thalidomide has proved effective in low-risk, mucocutaneous and/or bone forms of Langerhans cell histiocytosis, but it causes neurological adverse reactions of sedation and neuropathy [Citation7,Citation8]. Possessing improved potency and reduced side effects, lenalidomide is a functional and structural analog of thalidomide. Lenalidomide has been shown to exhibit anti-inflammatory, anti-angiogenic and immunomodulatory effects. Approved for treatments of multiple myeloma and myelodysplastic syndrome, its potential in resistant chronic lymphatic leukemia, follicular lymphoma, mantle cell lymphoma, diffuse large B-cell lymphoma and even solid tumor malignancies has also been described. But in contrast to thalidomide, myelotoxicity is the major dose-limiting side effect [Citation9,Citation10]. To the date, a MEDLINE literature search using key words lenalidomide and Langerhans cell histiocytosis has not revealed any reports on the application of this therapy in patients with LCH.
In conclusion, we are the first to report on a therapy effect of lenalidomide-based regimen in an adult patient with a repeatedly relapsed aggressive form of multisystem LCH with lymph node involvement. This new drug demonstrated a therapeutic potential in LCH resistant to some of the conventional treatments, while maintaining a high safety profile. Consequently, the patient has been controlled with lenalidomide for 11 months with a good quality of life free from any significant treatment related morbidity.
Acknowledgements
This work was supported in part by the IGA of The Ministry of Health (NT11154, NS10387, NS9671-4, FUNDIN MZ0MOU2005) and The Ministry of Education, Youth and Sports (LC06027, MSM0021622434).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Egeler RM, D'Angio GJ. Langerhans cell histiocytosis. J Pediatr 1995;127:1–11.
- Salotti JA, Nanduri V, Pearce MS, Parker L, Lynn R, Windebank KP. Incidence and clinical features of Langerhans cell histiocytosis in the UK and Ireland. Arch Dis Child 2009;94:376–80.
- Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol 1997;28:9–14.
- Howarth DM, Gilchrist GS, Mullan BP, Wiseman GA, Edmonson JH, Schomberg PJ. Langerhans cell histiocytosis: Diagnosis, natural history, management, and outcome. Cancer 1999;85:2278–90.
- Gotz G, Fichter J. Langerhans’– cell histiocytosis in 58 adults. Eur J Med Res 2004;9:510–4.
- Satter EK, High WA. Langerhans cell histiocytosis: A review of the current recommendations of the Histiocyte Society. Pediatr Dermatol 2008;25:291–5.
- McClain KL, Kozinetz CA. A phase II trial using thalidomide for Langerhans cell histiocytosis. Pediatr Blood Cancer 2007;48:44–9.
- Misery L, Larbre B, Lyonnet S, Faure M, Thivolet J. Remission of Langerhans cell histiocytosis with thalidomide treatment. Clin Exp Dermatol 1993;18:487.
- Palumbo A, Rajkumar SV. Multiple myeloma: Chemotherapy or transplantation in the era of new drugs. Eur J Haematol 2010;84:379–90.
- Vallet S, Palumbo A, Raje N, Boccadoro M, Anderson KC. Thalidomide and lenalidomide: Mechanism-based potential drug combinations. Leuk Lymphoma 2008;49:1238–45.