To the Editor,
The chemotherapeutic taxane docetaxel may induce diverse skin side effects that include hypersensitivity, fluid retention, infusion site reactions, alopecia, nail discoloration, onychodystrophy and onycholysis, photosensitivity, peripheral neuropathy and the acral erythrodysesthesia syndrome [also referred to as hand and foot syndrome (HFS) or palmar-plantar syndrome]. These reactions can be severe and debilitating and may provoke chemotherapy discontinuation. Acral erythrodysesthesia syndrome commonly occurs with several chemotherapeutic drugs, such as liposomal doxorubicin, capecitabine, infusional 5-fluorouracil, sorafenib, sunitinib and cytarabine but has been less often described in the literature in association with docetaxel. Here, we present a female patient with squamous cell esophageal cancer who developed HFS while receiving docetaxel.
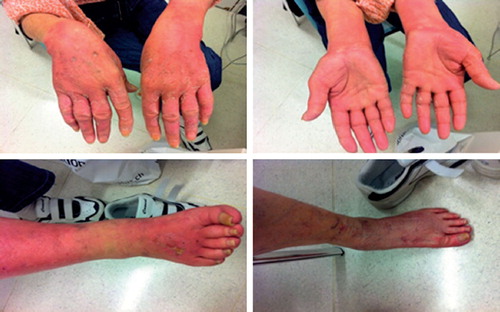
A 65-year-old woman with stage IB squamous cell esophageal cancer was started on treatment with induction chemotherapy by cisplatin 75 mg/m2 and docetaxel 75 mg/m2 every 21 days for two cycles, to be followed by definitive combined chemoradiation with weekly cisplatin and docetaxel as per a protocol of the Swiss Co-operative Oncology Group. Dexamethasone 8 mg was given orally twice daily for three days starting the day before chemotherapy for premedication. The patient received the first cycle without complications but two weeks after the second cycle, she developed erythema and pain of the palms and fingers of both hands and soles of both feet (). The erythema increased gradually and in the sixth week after docetaxel's first infusion, she developed burning and itching in the hands and feet and inability to walk due to severe pain followed by the occurrence of desquamation of fingers of both hands and the soles of both feet. Well-demarcated macules appeared in the dorsal surface of hands and feet ().
Figure 1. Erythema, desquamation and swelling over the hands after the second cycle of docetaxel. Rash in the dorsal surfaces of hands and feet
A punch biopsy of the skin revealed epidermal edema and a perivascular lymphocytic infiltration. There was a focal spongiosis in the basal stratum of the epidermis with isolated apoptotic keratinocytes.
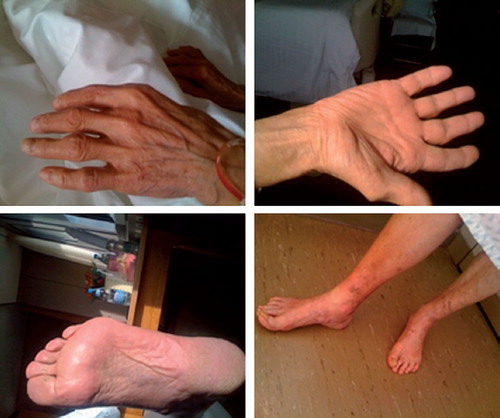
The patient was treated with emollient creams and topical steroids with gradual resolution of symptoms over three weeks (). Docetaxel was omitted and the patient received only weekly cisplatin at a dose of 25 mg/m2 in combination with radiotherapy.
Figure 2. Complete resolution of HFS three weeks after discontinuation of docetaxel and treatment with emollient creams and topical steroids.
Taxane drug docetaxel is a semisynthetic analog of paclitaxel. Both taxanes act as antimicrotubule agents causing cell cycle arrest with formation of microtubule bundles due to inhibition of tubulin depolymerization. Docetaxel is more avidly taken up by cells and is retained intra-cellularly for a longer time compared with paclitaxel and this difference may explain its different pharmacokinetics compared with paclitaxel [Citation1].
The pathogenesis of HFS related to docetaxel or to other chemotherapeutics is currently unknown. The most probable hypothesis remains a direct cytotoxicity to skin epithelial cells of acral areas. Palms and soles may be more sensitive to the drug due to weight bearing (feet) or use-related injury (hands). In addition, tiny capillaries in the palms and soles are believed to rupture under pressure from walking or use, releasing the cytotoxic agent and causing inflammatory reaction [Citation2]. That could explain why drugs with protracted serum levels, such as liposomal doxorubicin and capecitabine as well as docetaxel with increased intra-cellular retention are more likely to cause skin reactions [Citation2]. The fact that several drugs with different enzymatic metabolism can produce HFS makes an enzymatic deficiency cause less likely. Indeed the mechanism of HFS induced by different drugs may not be unique but instead differ in each instance. The clinical picture of HFS may also differ depending on the causative agent. A well-demarcated macular rash in the dorsal surfaces of hands and feet as seen in our patient is often seen with docetaxel HFS [Citation3,Citation4] but less commonly with other drugs. The new multi-tyrosine kinase inhibitors sunitinib and sorafenib-induced HFS has also some distinct clinical features with localized erythematous patches that may progress to bullae and a predilection for additional areas such as the lateral soles and inter-digital areas [Citation5].
Risk factors that increase docetaxel-related HFS occurrence are also unknown. Cumulative dose or concomitant diseases, such as diabetes or peripheral vascular atherosclerotic disease are not clearly predicting a higher risk. Steroids are commonly used as prophylactic premedication against the fluid retention syndrome and do not seem to protect against docetaxel induced skin toxicity. No standard therapy is definitively proven effective, but several symptomatic and prophylactic treatments have been used to alleviate symptoms including steroid ointments, occlusive dressings, COX-2 inhibitors, oral pyridoxine, blood flow reduction by local hypothermia with the use of frozen gloves, topical dimethylsulfoxide and oral vitamin E therapy [Citation5,Citation6]. The only proven strategy is treatment interruption but this has to be weighted against concomitant loss of treatment benefit.
Higher incidence of HFS is described during combination chemotherapy of 5-FU analogs and docetaxel. Our patient received a first line chemotherapy with docetaxel and cisplatin (an agent not commonly associated with HFS) and had no exposure to 5-FU analogs. Although no re-challenge with docetaxel was carried out the fact that HFS improved after docetaxel discontinuation while cisplatin was continued strongly suggests that the taxane was the responsible agent. The slightly different clinical picture of HFS according to inciting agent is of particular importance when two agents with HFS production potential are used concomitantly in order to determine the causing drug and modify treatment accordingly.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rowinski EK. Antimicrotubule agents. In: Chabner BA, Longo DL. Cancer chemotherapy and biotherapy. Lippincott Williams & Wilkins: Philadelphia, PA; 2006. pp. 237–82.
- Farhat MH, El-Saghir NS, Shamseddine AI. Hand-foot syndrome with docetaxel: A five-case series. Ann Saudi Med 2008;28:374–7.
- Eich D, Scharffetter-Kochanek K, Eich HT, Tantcheva-Poor I, Krieg T. Acral erythrodysesthesia syndrome caused by intravenous infusion of docetaxel in breast cancer. Am J Clin Oncol 2002;25:599–602.
- Childress J, Lokich J. Cutaneous Hand and Foot associated with cancer chemotherapy. Am J Clin Oncol 2003;26: 435–6.
- Lipworth AD, Robert C, Zhu AX. Hand-Foot Syndrome (Hand-Foot Skin Reaction, Palmar-Plantar Erythrodysesthesia): Focus on sorafenib and sunitinib. Oncology 2009; 77:257–71.
- Wilkes GM, Doyle D. Palmar-plantar erythrodysethesia. Clin J Oncol Nurs 2005;9:103–6.
