Abstract
Background. A prospective diagnostics and treatment protocol for extremity and trunk wall soft tissue sarcoma (STS) was introduced by the Scandinavian Sarcoma Group in 1986 and it was also widely adopted in Finland. We have updated the protocol and made it more detailed at the Helsinki University Central Hospital. We retrospectively compared diagnostics and treatment of STS in a nationwide population-based material to this protocol with special emphasis on local control. Methods. Data for 219 patients with an STS of extremity or trunk wall diagnosed during 1998–2001 was retrieved from the nationwide Finnish Cancer Registry. Histologic review was performed. Treatment centres were divided into high-, intermediate- and low-volume centres based on the number of patients with final surgery during the study period. Results. Significantly more patients were operated with a preoperative histological or cytological diagnosis at high-volume centres. No preoperative diagnosis was a strong predictor for the patient to undergo more than one operation (p < 0.0001). Wide surgical margin was achieved more often at high-volume centres, but in all centre categories a considerable percentage of patients with inadequate surgical margin did not receive adjuvant radiation therapy. Local control at five years was 82% at high-volume centres, 61% at intermediate-volume centres treating highest percentage of deep tumours and 69% at low-volume centres (p = 0.046). Local control improved as the number of patients operated (surgical volume of the centre) increased. Conclusion. The present quality-control study is the first nationwide population-based study to assess diagnostics and treatment of STS. When referred to a specialised sarcoma centre even patients with inadequate surgery can achieve good local control. STS is a rare cancer and its treatment should be centralised in Finland, which has 5.4 million inhabitants and approximately 100 new STSs of extremities and trunk wall annually.
Soft tissue sarcoma (STS) is a rare disease. The diagnostics and treatment of STS is highly demanding. The importance of centralisation of diagnostics and treatment of this neoplasm is well recognised [Citation1–5]. The Scandinavian Sarcoma Group (SSG) founded in 1979 recommends that all patients with deep tumours (tumours under the investing fascia or subcutaneous tumours with fascial infiltration) and patients with subcutaneous tumours larger than 5 cm should be referred to a sarcoma centre before any biopsy or surgery [Citation6,Citation7].
Several studies have shown shortages in referral and preoperative imaging in patients treated outside a tertiary sarcoma centre [Citation5] as well as in histopathological work-up [Citation8]. Wide margin was achieved in 66% of patients operated at a sarcoma centre and only in 11% operated outside a sarcoma centre [Citation5]. Only 48% of the patients received postoperative radiation therapy as recommended after intralesional or marginal surgery [Citation9]. None of the previous studies were nationwide and population-based.
The most important aim of the present study was to describe referral policy and treatment together with prognosis of STS in a first nationwide population-based material. Histologic review was also performed. SSG introduced treatment program for STS (SSG V) in 1986, and the protocol was widely adopted also in Finland. Our group has updated the protocol and made it more detailed. We have published several reports of STS treatment results at the Helsinki University Central Hospital (HUCH), for example adherence to treatment protocol [Citation3,Citation10], surgical outcome and survival [Citation11], importance of adequate surgical margin [Citation10] and feasibility of microvascular flaps in reconstruction after STS resection [Citation12]. In the present study, we compared diagnostics and treatment of STS nationwide to the treatment protocol used at HUCH, deriving from the SSG recommendations. The important aim was to compare treatment results by centres categorised as high, intermediate and low-volume centres based on surgical volume, with special emphasis on local control.
Material and methods
Data for patients diagnosed with a local soft tissue sarcoma of the extremity or trunk wall in Finland during 1998–2001 were retrieved from the nationwide population-based Finnish Cancer Registry. The time period of 1998–2001 was chosen in order to gain approximately 250 patients with a complete follow-up. The Finnish Cancer Registry was founded in 1952 and it covers the whole territory of Finland. The registry covers more than 99% of the solid tumours diagnosed in Finland [Citation13]. The study was approved by the Joint Ethics Committee of HUCH and by the Ministry of Health and Social Affairs. Detailed clinical data was collected from the patient files and survival data by using nationwide death records from the Population Register Centre.
Setting
Finland (with an area of 338 400 km2 and a population of 5.4 million) has approximately 300 primary health care centres with mainly general practitioners, 51 district and central hospitals, and five university hospitals. No exact public data was available for the number of private practitioners. HUCH has for long been responsible for challenging STS treatment in Finland.
Treatment protocol
Referral. All patients with a deep tumour and patients with a subcutaneous tumour larger than 5 cm are recommended to be referred to a university hospital before any biopsy or surgery. Patients who have undergone a biopsy or non-radical surgery should be referred to a university hospital when a sarcoma diagnosis is confirmed.
Preoperative investigations. Tumour size and depth should be evaluated and reported. Preoperatively patients should undergo an magnetic resonance imaging (MRI) or a computed tomography (CT) scan of the primary tumour. A core needle biopsy and/or a fine needle aspiration should be performed at the centre responsible for the definite surgery. A CT-scan of the lungs should be performed preoperatively in patients with a high-grade tumour.
Surgery. Surgical resection with wide margin is the primary treatment in all cases if possible without major sacrifice of function. A reoperation is recommended after intralesional surgery, if feasible.
Pathology report. Tumour size (cm) as the largest diameter of the tumour in the surgical specimen is to be reported together with the histologic grade in a four-tiered scale modified from Broders et al. [Citation14] and Angervall et al. [Citation15]. Grades 1 and 2 are considered low and grades 3 and 4 are considered high grade. The smallest surgical margins in soft tissue not limited by a fascia should be evaluated on histological slides of the resection specimen and reported. A margin of at least 2.5 cm is defined as wide. Narrower margin is accepted if it contains uninvolved anatomical barrier, for example fascia. The presence of necrosis and vascular invasion should be reported. In the present review, tumour size and smallest margin were recorded from the original pathology reports and were not re-evaluated.
Radiation therapy. Radiation therapy is recommended after marginal or intralesional surgery. Radiation therapy was initially recommended with the radiation dose of 50 Gy in five weeks (2 Gy/day). For microscopically or macroscopically involved surgical margins, a boost was recommended to a smaller target volume (10–20 Gy in one to two weeks). At the beginning of 2000 the protocol was modified with a recommendation of a boost also after marginal surgery.
Chemotherapy. Starting from 1998 adjuvant doxorubicin-based chemotherapy was recommended for all patients less than 70 years and with sufficient performance status with a high-grade tumour if the tumour fulfills at least two of the following criteria: size greater than 8 cm (in synovial sarcomas 5 cm), necrosis, or vascular invasion.
Follow-up. Patients undergo a regular follow-up. For high-grade sarcomas, the interval is two months during the first two years, thereafter three times annually up to five years. Follow-up is extended in patients with synovial sarcomas to 10 years with annual visits up to seven years and thereafter every 18 months. Patients undergo a chest x-ray at each visit. An MRI or CT-scan of the operative area is taken six months postoperatively and every six months up to two years and thereafter once annually up to five years, and for patients with synovial sarcomas annually up to seven years after five years and thereafter once in every 18 months up to 10 years.
For low-grade tumours, the interval is three times annually up to two years and thereafter two times a year up to five years, annually up to seven years and thereafter once in every 18 months up to 10 years. Patients undergo a chest x-ray at each visit. An MRI or CT-scan of the operative area is taken annually up to seven years and thereafter once in every 18 months up to 10 years.
Statistical methods
For the survival rate comparisons, patients were divided into groups based on the hospital in which they had their final surgery and decision on adjuvant therapy. The largest centres treating two thirds of the patients (of the final surgeries) during the study period were arbitrarily defined as “high-volume” centres (three university hospitals). Sixteen hospitals treated only one to two patients during the study period and were defined as “low-volume” centres. Five hospitals (two university hospitals and three district hospitals) with 3–17 patients treated were defined as “intermediate-volume” centres. Local recurrence-free rates, metastases-free rates and sarcoma-specific survival rates were calculated with the Kaplan-Meier method. Differences in survival rates were analysed with the log-rank test. Relative risks (RR) for survival rates in the centre volume analysis were calculated for an increment of 10 patients using Cox's proportional hazards model. The χ2 test and Fisher's exact test were used to assess differences in the distribution of tumour and treatment characteristics among subgroups. The level of significance was set at p < 0.050. SPSS® PASW (Predictive Analytics SoftWare) Statistics for PC, version 18.0, was used for all analyses.
Results
Patients with dermatofibrosarcoma protuberans (n = 74), grade I liposarcoma/atypical lipoma (n = 31) and cutaneous leiomyosarcoma (n = 6) were excluded from the analysis. Eight patients were excluded because of missing files. We also excluded patients who received treatment with palliative intention (n = 10) leaving 219 patients with local disease to the final analysis. The cytological or histological diagnosis was made at high-volume centres in 163 (74%) of the 219 tumours. The original histologic slides of the resection specimen of 200 (91%) patients were available for review (). Also patients with missing original slides were included in further analysis. Four sarcoma diagnoses were corrected in the review to benign tumours or tumours with borderline malignant potential and were excluded from further analysis ().
Table I. Initial diagnoses (n = 219) and histological review of the 200 cases available.
The histologic subtype was corrected in 20 (10%) and the grade in eight (4%) of the 200 diagnoses in the re-evaluation. Both the grade and the subtype were corrected in two tumours. The diagnoses were corrected for 9% of the tumours assessed at high-volume centres and for 28% of the tumours assessed at intermediate or low-volume centres. The surgical treatment and decision of adjuvant radiation therapy were irrespective of the sarcoma subtype and grade so these changes would not have had any impact to the treatment of choice. Two patients with a benign tumour or a tumour with borderline malignant potential received unnecessary radiation therapy; otherwise the treatment was appropriate based on the histologic review. The smallest margin in centimeters was not reported in 40 tumours: 28 were reported to have a marginal margin and 12 a wide margin. Higher percentages of deep tumours and younger patients were treated at centres with high or intermediate volumes, otherwise distributions of patient and tumour characteristics were similar ().
Table II. Characteristics of patients and tumors treated at HVCs, IVCs, and LVCs.
shows adherence to guidelines for preoperative investigations. Only 96 (42%) patients had their first operation at a high-volume centre. The recommended preoperative staging investigations and biopsies were performed significantly less often at intermediate and low-volume centres.
Table III. Preoperative investigations.
shows adherence to the treatment guidelines. If a preoperative histological or cytological diagnosis was not made, this was a very strong predictor for the patient to undergo multiple operations: of the 96 patients with no preoperative biopsy, 75 (78%) underwent another operation and six patients (6%) underwent two more operations. Primary surgeries were performed in 53 institutions and definite surgeries in 23 institutions. Three university hospitals were high-volume centres with 20–105 new patients during the whole study period. Four hospitals were intermediate-volume centres with 3–17 new patients during the study period. Sixteen hospitals treated only one or two new patients during the study period (low-volume centres).
Table IV. Treatment characteristics.
Definite surgery was performed and adjuvant therapy planned for 153 (71%) patients at high-volume centres. The percentage of patients not operated on at a university hospital varied from 4% to 36% in the five university hospital dictricts (p < 0.0001). A wide final margin was achieved significantly more often at high-volume centres, 31.4% vs. 17.5% and 14.2%, respectively. The patients with an inadequate margin received radiation therapy significantly more often at high-volume centres, 75.2% vs. 56.3% and 31.6%, respectively.
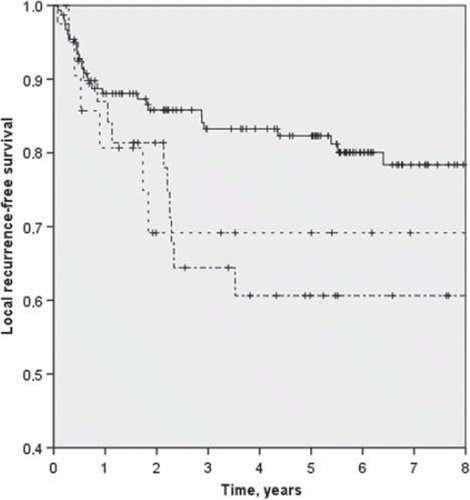
The local recurrence-free rate at five years was significantly higher at high-volume centres than at intermediate and low-volume centres, 82% vs. 61% and 69% (p = 0.046) (). The sarcoma-specific survival was higher at high-volume centres, although the difference was not statistically significant, 71% vs. 59% and 66% (p = 0.237). The metastases-free survival was 67% at high-volume centres and 61% and 78% at intermediate and low-volume centres, respectively (p = 0.283). The local-recurrence rate decreased as the surgical volume of the centre increased: RR per 10 patients was 0.914 (95% CI 0.851–0.970; p = 0.006). For metastases-free survival (RR 1.000; 95% CI 0.951–1.051; p = 0.982) and sarcoma-specific survival (RR 0.961; 95% CI 0.914–1.010; p = 0.111) it had no effect.
Discussion
To our knowledge this is the first population-based, nationwide quality-control study on referral policy and treatment results of STS. We compared diagnostics and treatment retrospectively to the treatment protocol introduced in 1986 by the Scandinavian Sarcoma Group. The protocol was widely adopted also in Finland and further developed and updated by the STS group at HUCH. In the present study, we compared the treatment results of the largest centres named “high-volume” centres by surgical volume to smaller centres entitled “intermediate-volume” centres and “low-volume” centres. Division was arbitrarily made with a cut-off point of high-volume centres treating two thirds of the patients. Although named “high-volume” centres the number of patients treated in these centres annually was as low as five to 25 during 1998–2001. Hospitals treating only an occasional sarcoma (one to two during the study period) were defined as “low-volume” centres and the rest as “intermediate-volume” centres. Intermediate-volume group was introduced as it was expected that these larger centres were more experienced with this rare disease than low-volume centres. We are, however, unable to provide any data on surgeon case loads. In larger centres, there are usually several plastic surgeons or orthopaedic surgeons who operate on STS patients. On the contrary, in smaller centres, there may be only one surgeon treating soft tissue tumours.
Seventy-four percent of histopathological diagnoses were made at high-volume centre pathology laboratories meaning that the histopathologic diagnostics was well centralised. The subtype or the grade was corrected in 13% of the tumours in the present histologic review. Randall et al. [Citation8] presented a 104-patient series in which 37% of diagnoses were corrected in review. In our series radiation therapy would have been omitted from two patients with a benign or borderline tumour in case of correct evaluation at diagnosis. Surgical margin in centimeters was not reported in 18% of the reports.
Radiation therapy improves local control after inadequate surgery but administration of adjuvant radiation therapy was suboptimal in all categories of treatment centres. A patient-related reason for withdrawal of radiation therapy was infrequent. Instead, the most frequent reason was low malignancy grade and an expected low local recurrence rate. One probable reason for undertreatment was also the incompleteness of the pathology reports. The surgical margin was not reported in centimeters for 40 patients. Of these, 12 patients had a wide surgical margin reported and 28 a marginal margin. Eight of these patients received radiation therapy. It is likely that many of these patients had inadequate margins in regard to our treatment protocol and would have benefited from adjuvant radiation therapy. Only 45% of patients with marginal or intralesional margins received postoperative radiation therapy in the review from Scandinavian Sarcoma Group Registry [Citation5]. In the French review the number was 48% [Citation9].
Definite surgery and decision of adjuvant therapy was performed in three high-volume centres among 71% of patients and in 20 other hospitals among 29% of patients. Sixteen low-volume centres treated only one or two patients during the whole study period. Lack of experience caused high number of operations without proper preoperative investigations, that is, radiological imaging and histological or cytological biopsy. Surgeries at low-volume centres were mostly performed by district hospital surgeons and GPs in primary health care centres. Most of these patients needed further surgery.
Previous inadequate local treatment makes the second operation more difficult based on changed anatomy, tumour spreading and scarring. It is thus also more difficult to achieve good local control [Citation16]. We have however shown that patients with locally recurrent disease at presentation can have similar local control to patients with primary tumour with aggressive surgical treatment and radiation therapy [Citation10]. Local control at high-volume centres was superior to local control rates at intermediate-volume and low-volume centres in the present study (p = 0.046). It is noticeable that over one third of patients receiving definite surgery at high-volume centre were first treated at intermediate or low-volume centres and referred after the diagnosis of STS for further surgery. Results are in conjunction with the results in SSG series reflecting uniform recommendations for STS treatment in Scandinavia [Citation5,Citation17]. Local control at intermediate-volume centres was lowest most likely because of higher percentage of deep-seated tumours and shortages in radiation therapy administration. In the present study, low-volume centres had better local control than intermediate-volume centres mainly because of higher percentage of superficial tumours where local control is easier to achieve. Numbers were also small. As published also by Nijhuis et al. [Citation18] patients not referred to a specialised sarcoma treating team were older than the patients referred. Distribution of prognostic factors was otherwise identical.
Surgical volumes in Finnish institutions were low as the number of new patients treated annually was equal or more than five only in three of 23 institutions with definite surgical procedures. In a state-wide analysis from Florida, USA treatment of STS at a high-volume centre (more than four new patients annually in average) was a significant independent predictor of improved survival (OR = 1.292, p = 0.047) [Citation19]. The conclusion was that patients with STS should be treated at high-volume centres for better survival and functional outcome. Patients with either large (>10 cm), high-grade or truncal/retroperitoneal tumours should be treated exclusively at high-volume centres. In the present study, there was no significant survival benefit from treatment at the high-volume centres.
Although HUCH is the largest tertiary sarcoma referral centre in Finland, the annual number of new patients with a limb or trunk wall STS was approximately 26 during 1998–2001. Nowadays it is approximately 50, that is, half of the new soft tissue sarcomas of the extremity or trunk wall diagnosed annually in Finland. Unfortunately we have no data at the moment if these “new” patients are referred from low-volume or intermediate-volume centres as defined in the present study. That is a subject for a future study. Local control improved in the present study as the surgical volume of the hospital increased. Higher annual surgeon case-load was associated with decreased morbidity and mortality in colon cancer [Citation20] and with decreased operative mortality in pancreatic and oesophageal cancer [Citation21] even after stratified for hospital volume. Higher annual surgeon case-load decreased surgical mortality in patients undergoing gastrectomy for cancer [Citation22] and postoperative morbidity after prostatectomy [Citation23]. Patients with diagnosed breast cancer were recommended to be treated by surgeons treating over 30 breast cancer patients annually [Citation24]. No such data were available for STS. Sarcoma surgery is challenging because of local infiltrative growth, often a large tumour size and location anywhere in the body making each operation different. That calls for surgeons’ experience with this rare disease. With so few annual cases centralisation is warranted in order to maintain high-quality surgery and ascertain training.
The present study is the first nationwide population-based analysis on treatment results of STS. However, it suffers from several weaknesses. First and most importantly, the study was not randomised. It is probable that patients with poor physical performance status and/or elderly patients are not referred to specialised sarcoma centres. Instead, they have less radical treatment in the local hospital regarded in the present study as low-volume centres. One potential cause of selection bias are regional referral policies which was evident as in the five university hospital districts the percentage of patients not operated on at a university hospital varied from 4% to 36%.
In conclusion, multimodality treating teams are of paramount importance in rare diseases, such as STS. It is probable that further centralisation would standardise decision-making on diagnostics and treatment and improve treatment results.
Acknowledgements
The study was supported by EVO funds, Helsinki University Central Hospital and Finnish Cancer Society. Dr Sampo was supported by a grant from Finska Läkaresällskapet and Duodecim. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Gustafson P. Soft tissue sarcoma. Epidemiology and prognosis in 508 patients. Acta Orthop Scand Suppl 1994;259:1–31.
- Karakousis CP, Proimakis C, Walsh DL. Primary soft tissue sarcoma of the extremities in adults. Br J Surg 1995;82:1208–12.
- Wiklund T, Huuhtanen R, Blomqvist C, Tukiainen E, Virolainen M, Virkkunen P, . The importance of a multidisciplinary group in the treatment of soft tissue sarcomas. Eur J Cancer 1996;32A:269–73.
- Clasby R, Tilling K, Smith MA, Fletcher CD. Variable management of soft tissue sarcoma: Regional audit with implications for specialist care. Br J Surg 1997;84:1692–6.
- Bauer HC, Trovik CS, Alvegard TA, Berlin O, Erlanson M, Gustafson P, . Monitoring referral and treatment in soft tissue sarcoma: Study based on 1,851 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand 2001;72:150–9.
- Stener B. The management of soft tissue tumors. Int Orthop 1978;1:289–98.
- Rydholm A. Management of patients with soft-tissue tumors. Strategy developed at a regional oncology center. Acta Orthop Scand Suppl 1983;203:13–77.
- Randall RL, Bruckner JD, Papenhausen MD, Thurman T, Conrad EU, 3rd. Errors in diagnosis and margin determination of soft-tissue sarcomas initially treated at non-tertiary centers. Orthopedics 2004;27:209–12.
- Ray-Coquard I, Thiesse P, Ranchere-Vince D, Chauvin F, Bobin JY, Sunyach MP, . Conformity to clinical practice guidelines, multidisciplinary management and outcome of treatment for soft tissue sarcomas. Ann Oncol 2004;15:307–15.
- Sampo M, Tarkkanen M, Huuhtanen R, Tukiainen E, Bohling T, Blomqvist C. Impact of the smallest surgical margin on local control in soft tissue sarcoma. Br J Surg 2008;95:237–43.
- Popov P, Tukiainen E, Asko-Seljaavaara S, Huuhtanen R, Virolainen M, Virkkunen P, . Soft tissue sarcomas of the lower extremity: Surgical treatment and outcome. Eur J Surg Oncol 2000;26:679–85.
- Barner-Rasmussen I, Popov P, Bohling T, Tarkkanen M, Sampo M, Tukiainen E. Microvascular reconstruction after resection of soft tissue sarcoma of the leg. Br J Surg 2009;96:482–9.
- Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol 1994;33:365–9.
- Broders AC, Hargrave R, Meyerding HW. Pathologic features of soft tissue fibrosarcoma with special reference to the grading of its malignancy. Surg Gynecol Obstet 1939;69:267–80.
- Angervall L, Kindblom LG, Rydholm A, Stener B. The diagnosis and prognosis of soft tissue tumors. Sem Diagn Pathol 1986;3:240–58.
- Robinson M, Barr L, Fisher C, Fryatt I, Stotter A, Harmer C, . Treatment of extremity soft tissue sarcomas with surgery and radiotherapy. Radiother Oncol 1990;18:221–33.
- Gustafson P, Dreinhofer KE, Rydholm A. Soft tissue sarcoma should be treated at a tumor center. A comparison of quality of surgery in 375 patients. Acta Orthop Scand 1994;65:47–50.
- Nijhuis PH, Schaapveld M, Otter R, Hoekstra HJ. Soft tissue sarcoma – compliance with guidelines. Cancer 2001; 91:2186–95.
- Gutierrez JC, Perez EA, Moffat FL, Livingstone AS, Franceschi D, Koniaris LG. Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg 2007;245:952–8.
- Drolet S, MacLean AR, Myers RP, Shaheen AA, Dixon E, Buie WD. Elective resection of colon cancer by high-volume surgeons is associated with decreased morbidity and mortality. J Gastrointest Surg 2011;15:541–50.
- Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117–27.
- Hannan EL, Radzyner M, Rubin D, Dougherty J, Brennan MF. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery 2002;131:6–15.
- Begg CB, Riedel ER, Bach PB, Kattan MW, Schrag D, Warren JL, . Variations in morbidity after radical prostatectomy. N Engl J Med 2002;346:1138–44.
- Sainsbury R, Haward B, Rider L, Johnston C, Round C. Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet 1995;345:1265–70.