To the Editor,
Glioblastoma multiforme (GBM) is the most aggressive subtype of malignant gliomas. It is also one of the most vascularized tumors. Despite aggressive surgery, radiotherapy, and chemotherapy regimens, median survival is less than 15 months. The use of radiotherapy with concurrent temozolomide (75 mg/m2/day) as well as temozolomide alone (150–200 mg/m2 for five days every four weeks) is currently the standard therapy for GBM [Citation1]. Despite the improved survival, the rate of recurrence is very high.
Bevacizumab, a monoclonal antibody against the vascular endothelial growth factor (VEGF), is a potent inhibitor of angiogenesis. It binds and inactivates VEGF, thereby inhibiting endothelial cell activation and tumor proliferation [Citation2]. Herein we report a complete remission after using the bevacizumab and temozolomide combination therapy in a patient with recurrent GBM. However, bevacizumab was discontinued during the follow-up due to bilateral visual loss.
Case
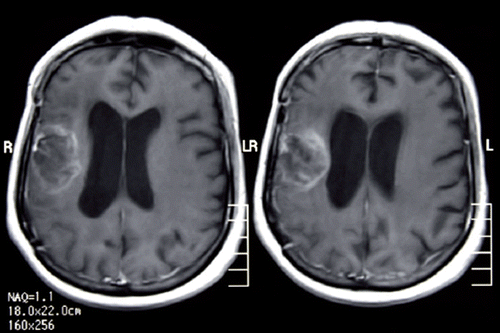
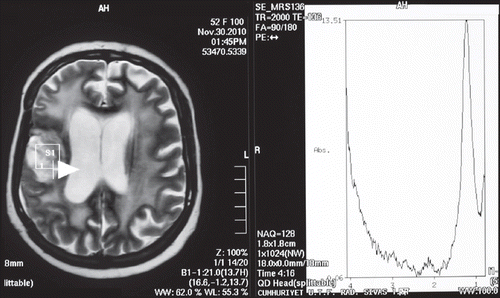
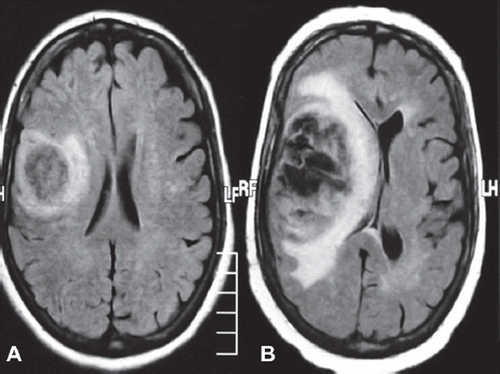
A 54-year-old woman admitted with hypoesthesia at her left arm and face in August 2009. Her medical history revealed hypertension for the last five years. A left-sided weakness and decrease in mobility were observed in her physicial examination. Cranial magnetic resonance imaging (MRI) revealed a mass of 25 × 20 mm involving the right frontotemporal lobe with additional peritumoral vasogenic edema (). Subsequently, the mass was totally excised. Histochemical examination confirmed the diagnosis of GBM. She was treated with postoperative cranial radiotherapy (total 60 Gy) with concurrent temozolomide (75 mg/m2/day). At the follow-up, due to the developing persistant thrombocytopenia, temozolomide was discontinued. Two months after radiotherapy, temozolomide was restarted at a dose of 200 mg/m2/day for five days for every four weeks. Yet, the disease progressed after two cycles of the chemotherapy (). However, because the patient was inoperable, bevacizumab (10 mg/kg every two weeks; total dose: 900 mg/day) was added to temozolomide. After three months, cranial MRI revealed a prominent regression of the intracranial mass. She was again mobilized with significantly improved neurological findings. At six months after the therapy, postcontrast axial T1-weighted MR images revealed a heterogenous contrast-enhancing lesion at the right frontal lobe (). MR spectroscopy of the patient revealed undetectable peak intensities of choline, creatine and N-asetil aspartate, with a significantly increased lactate peak (). These findings were consistent with radiation necrosis. At 10 months after the initiation of the combination therapy, she presented with a painless loss of vision in her left eye. There were no other systemic symptoms. Her blood pressure was within normal ranges. During the follow-up, she also developed a loss of vision in the right eye. Her visiual activity was found as 1/10 (right eye) and 0/10 (left eye). Bilateral funduscopic examination revealed bilateral optic atrophy. There were no new lesions, retinal edema or hemorrhage, with no progression according to the cranial and bilateral orbital MR images. Consequently, the combination therapy involving bevacizumab and temozolomide was discontinued and dexamethasone was initiated at a dose of 8 mg bid. The patient was still alive in October 2011 without any progression.
Figure 1. A. Magnetic resonance imaging of the primary tumor at the baseline; B. progressive disease after two cycles of Temozolomide therapy.
Discussion
During the last decade, much advancement in the treatment of GBM has been recorded. First, temozolomide, an orally administered well-tolerated DNA alkylating agent, was reported to improve the median survival of the patients, when given concomitant with or after radiotherapy. The five-year overall survival rate was superior with temozolomide and radiotherapy compared to radiotherapy alone (10% vs. 2%, respectively). However, there has yet been no standard therapy regimens described in case of the recurrence of the disease. For the treatment of GBM recurrence, medications like bevacizumab and irinotecan are still under investigation in many different clinical trials.
The first data in favor of bevacizumab in the treatment of recurrent GBM was reported in 2005 in 21 patients with malignant glioma [Citation3]. Due to the higher response rates observed with bevacizumab, subsequent clinical trials were designed. In a recent study, a response rate of 35% was documented with bevacizumab. Thus, the use of bevacizumab was approved by FDA as a monotherapy for patients with GBM who progressed after the initial therapy [Citation4,Citation5]. In addition to its clinical benefits, bevacizumab-based therapies provided symptomatic relief and improvement in patients with recurrent malignant glioma [Citation6].
Despite improved response rates and survival, bevacizumab was documented to have a number of side effects including hypertension, proteinuria, impared wound healing, thrombosis, and hemorrhage. Thrombosis and hemorrhage are the most frequently observed bevacizumab-related adverse events and these side effects are the most common cause for the discontinuation of this agent. The rate of thrombosis was reported as 12.5% in patients with GBM who were treated with bevacizumab [Citation4].
Sherman et al. reported the development of severe optic neuropathy in six patients with GBM receiving bevacizumab [Citation7]. However, it should be noted that those patients in their study also received cranial radiotherapy with concomitant temozolomide. Median dose of bevacizumab before the onset of visual symptoms was 7.5 (3–18) in those patients. Visual loss was reported to appear bilaterally in three patients and cerebrospinal fluid analysis was unremarkable in any of the cases. Moreover, only in two patients MR images revealed T1 enhancement in optic nerves.
Visual loss in patients with GBM might be explained with several mechanisms. A major mechanism which is believed as the most common cause of this adverse event can be the compression and progression of the primary tumor. Another possible mechanism is radiotherapy-induced necrosis and optic nevre damage. It was reported, however, that bevacizumab-related thrombosis and hemorrhage were the main causes of visual loss in patients treated with this agent. Last but not the least, optic neuropathy that might develop after bevacizumab treatment can cause visual loss.
In the subject of this case report, the disease was not progressive. Although radiotherapy-induced optic atrophy occurs usually bilateral, in this case, the loss of vision first appeared in the left eye and three weeks after in the right eye. Nevertheless, optic neuropathy could not be excluded in the patient. In conclusion, it is highly recommended that physicians should keep the possibility of the development of optic atrophy and/or visual loss following bevacizumab treatment in mind.
Declaration of interest: The authors declare that they have no competing interests.
References
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96.
- Burger RA. Experience with bevacizumab in the management of epithelial ovarian cancer. J Clin Oncol 2007;25: 2902–8.
- Stark Vance V. Bevacizumab (AvastinR) and CPT-11 (CamptosarR) in the treatment of relapsed malignant glioma. Neurooncol 2005;7:369 (abstract).
- Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, . Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009;7:740–5.
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, . Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009;27: 4733–40.
- Hofer S, Elandt K, Greil R, Hottinger AF, Huber U, Lemke D, . Clinical outcome with bevacizumab in patients with recurrent high-grade glioma treated outside clinical trials. Acta Oncol 2011;50:630–5.
- Sherman JH, Aregawi DG, Lai A, Fathallah-Shaykh HM, Bierman PJ, Linsky K, . Optic neuropathy in patients with glioblastoma receiving bevacizumab. Neurology 2009; 73:1924–6.