Abstract
Introduction. Estrogen receptor (ER) status is not an optimal marker for response to adjuvant endocrine therapy since approximately 30% of patients with ER-positive tumors eventually relapse. Bcl-2 is regulated by ER and may thus be considered as an indicator of ER activity and a candidate supplementary marker to ER status. Patients and methods. Tumor tissue from 257 patients with ER-positive breast cancer treated with tamoxifen was used for determination of the best threshold for immunohistochemical Bcl-2 assessment as prognostic marker. Subsequently, samples from the Danish patients of the randomized clinical trial BIG 1-98 comprising 1191 ER-positive patients treated with tamoxifen, letrozole or a sequence of the two were immunohistochemically stained for Bcl-2 to further explore the prognostic value of Bcl-2. Results. Two Bcl-2 levels were found to divide the population of the primary study into significantly different groups according to disease-free survival (DFS). Multivariate analysis confirmed the significance of the lowest level, and showed Bcl-2 to be an independent prognostic marker. Analysis of the Danish cohort of the BIG 1-98 confirmed that Bcl-2 was a significant predictor of DFS, independent of known prognostic markers. However, in an additional analysis of a subset of the Danish cohort of BIG 1-98 including only HER-2 normal patients, the effect of Bcl-2 was not statistically significant. Discussion. Low Bcl-2 can predict poor outcome of patients with ER-positive tumors treated with adjuvant endocrine therapy, whereas the use of Bcl-2 for determination of addition of chemotherapy was not supported by this study.
Adjuvant endocrine treatment improves the survival of patients with estrogen receptor (ER)-positive tumors. Nevertheless, 30% of the patients who received adjuvant tamoxifen therapy relapsed during the 10-years postoperative period [Citation1]. Therefore, it is important to identify patients with endocrine- resistant breast cancer, since alternative or additional treatments with chemotherapy, other kinds of endocrine treatment, or targeted agents may be more effective. Currently, ER is the only marker used routinely to predict endocrine response [Citation1,Citation2], but much effort is devoted to finding new markers.
Bcl-2 is an antiapoptotic protein [Citation3] that is upregulated by estrogen stimulation [Citation4] and reported to be overexpressed in 28–80% of breast cancer patients, when using different cutpoints for dichotomous assessment of positive status [Citation5–10]. Bcl-2 expression correlates with clinical prognostic markers, such as small tumor size and low tumor grade [Citation5,Citation11], low proliferation rate, ER positivity and weak or absent p53 [Citation9–12] and has also been shown to have prognostic value as recently confirmed in a large meta-analysis by Dawson et al. [Citation7].
We hypothesize that since Bcl-2 expression depends on functional ER, the expression level of Bcl-2 will supplement the ER measurement in estimating endocrine responsiveness, resulting in a worse prognosis for patients with tumors having low levels of Bcl-2.
To assess whether a given protein in a malignant tumor has prognostic and/or predictive properties, it is important, in advance, to determine a valid threshold where the tumor is defined as negative or positive. Furthermore a specific subgroup of tumors, like the ER-positive tumors, might have properties that influence the expression of a marker and hereby influence the threshold for prognostic value of that marker. Therefore, the threshold should be defined in a clinical setting and related to the outcome of the relevant patient group. In the present study we investigated the prognostic value of Bcl-2 in the adjuvant setting of endocrine treatment including finding the best threshold for Bcl-2 in two independent study populations.
Patients and methods
Patients
This study included two comparable but independent populations of breast cancer patients. One cohort was used for defining the best threshold for Bcl-2 positivity, and another cohort, the Danish patients in the BIG 1-98 study, served to validate the results.
The first cohort (called Odense) was a retrospective cohort of 257 high-risk, post- or perimenopausal patients who between 1989 and 2001 had breast conserving surgery or mastectomy at Odense Hospital, Denmark. The patients were treated with tamoxifen as adjuvant treatment according to guidelines of the Danish Breast Cancer Cooperative Group (DBCG) [Citation13]. The allocated duration of tamoxifen treatment was 0.5, 1, 2, or 5 years. High-risk criteria included one of the following: positive axillary lymph nodes, tumor size >50 mm (>20 mm since 1999) or ductal grade II–III (since 1999). Additional criteria for inclusion was age at surgery less than 75 years (<70 years until 1989). All patients had positive or unknown status of ER and/or progesterone receptor (PgR). Patients were selected on the basis of the availability of fresh, frozen tumor tissue from a total cohort of 589 patients; patients with unknown or negative ER status (<1% positive cells) at central reassessment were excluded.
BIG 1-98 Danish cohort:
The BIG 1-98 trial was a randomized, phase 3, double-blinded clinical investigation of hormone receptor-positive breast cancer in postmenopausal women [Citation14]. Patients were enrolled from 1998 to 2003. Treatment arms were: tamoxifen or letrozole for five years, two years of tamoxifen or letrozole followed by three years of letrozole or tamoxifen. In total, 1396 Danish patients were randomized in BIG 1-98. Primary tumor samples from 1323 patients were suitable for tissue microarray (TMA) preparation and 1301 samples were available for immunohistochemical (IHC) staining. Patients with ER-negative tumors (<1% positive cells), found in the central evaluation of the present study, and patients with unknown ER status were excluded from the analysis (n =23 and n =5); 82 additional patients were excluded because tissue was missing in TMA slides for Bcl-2 evaluation. The remaining 1191 patients were included in the analyses. In an additional analysis, patients with human epidermal growth factor receptor 2 (HER-2) amplification (n =109) were further excluded from the study population since HER-2 is a negative prognostic marker that often requires chemotherapy irrespective of other markers. The study has been approved by the local ethics committees: The Danish National Committee on Biomedical Ethics in Denmark (BIG 1-98 trial in 1997 (KF 02-178/97) with the current biomarker study as an addendum approved in 2004 (KF 12-142/04)) and The Region South Denmark (S-VF-20040064).
Tissue microarray construction
Archival formalin-fixed and paraffin-embedded primary tumor tissue was used to generate TMAs from both studies comprising two, 2 mm cores from each tumor using a TMA builder (AH diagnostics) [Citation15].
Immunohistochemistry and fluorescence in situ hybridization (FISH) analyses
Sections cut from the TMA blocks were stained for Bcl-2, ER, PgR, HER-2 and Ki-67. All protocols used were part of routine clinical testing.
Supplementary Table I to be found online at www.informahealthcare.com/doi/abs/10.3109/0284186X.2011.653009 shows details about antibodies, dilutions, retrieval methods and visualization systems used. HER-2 IHC results scored as 2 + was further analyzed by FISH as recommended by Herceptest Guidelines. A HER-2/centromere ratio of ≥2.0 was considered as HER-2 gene amplification. Herceptest staining, FISH procedure and resulting evaluation were performed according to the manufacturer's manual in both studies.
Evaluation of immunohistochemistry
At the time of the IHC evaluation, investigators were blinded to the clinical outcomes.
The evaluation of the IHC staining for Bcl-2 in the Odense cohort was performed by two pathologists, independently. Cases of disagreement were reviewed jointly to reach a consensus score. ER, HER-2, PgR and Ki-67 IHC were scored by one of the pathologists (MSL or A-VL) or a trained observer (KLH). One or two cores were scored. The vast majority of cases had identical score values. If score values differed, the higher value was used for Bcl-2, Ki-67 and HER-2, whereas the median value was used for ER and PgR.
Bcl-2 staining was assessed as the percentage of stained tumor cells (<1%, 1–9%, 10–49%, 50–100%) combined with staining intensity (weak, moderate, strong). A combined classification of Bcl-2 was subsequently made: 0–9%, 10–49%, 50–100% weak, moderate, or strong intensity. ER and PgR were assessed as percentage positive tumor cells in a category of less than 1% and thereupon categories of 10% percentiles. For Ki-67 staining the total fraction of tumor cells expressing any nuclear Ki-67 reaction was counted.
HER-2 was scored according to the guidelines for HER-2 staining as 0, 1 +, 2 + or 3 +.
In subsequent analyses, ER was grouped as 1–49%, 50–79% and 80–100%, while PgR, Ki-67 and HER-2 were dichotomized where <1% (PgR), <14% (Ki-67) and scores 0, 1 +, 2 +/negative FISH (HER-2) were interpreted as negative.
Clinical data
For the Odense cohort, data on primary patient characteristics and follow-up until 10 years after surgery were obtained from the DBCG-registry [Citation13] and was supplemented by local examination of medical records and by record linkage to the Danish Central Population Registry (date of death or emigration). For the Danish BIG 1-98 population, clinical data and follow-up data were obtained from the International Breast Cancer Study Group (IBCSG) Statistical Center.
Endpoint
In the Odense cohort disease-free survival (DFS) was defined as: the time from surgery, and in BIG 1-98, as time from random assignment to the earliest of invasive recurrence in local, regional, or distant sites, new contra-lateral invasive breast cancer, new secondary non-breast malignancy, or death from any cause. For the Odense cohort, observations of follow-up were censored at emigration, 10 years after surgery, or April 1, 2008, whichever came first. For the BIG 1-98 population, observations of follow-up were censored at the date of last patient contact before the data cut-off date of July 2008.
Statistics
Inter-observer agreement on Bcl-2 score was determined as percentage agreement and systematical bias in non-agreement scores was assessed by a binomial sign test. The association between Bcl-2 and prognostic markers was assessed by Wilcoxon rank-sum test. Proportions were tested by Fisher's exact test.
Follow-up time and DFS according to Bcl-2 score was estimated by the Kaplan-Meier (KM) method and statistical significance was estimated by using the log-rank test. Cox proportional hazard regression models were used to examine the association between DFS and Bcl-2 and other prognostic markers. The proportional hazards assumption was investigated by Schoenfeld residuals. When non- proportional hazard rates were found, time dependent variables were constructed or the variable was included as a stratification factor. Models used in analyses of the Odense cohort were stratified by the allocated duration of tamoxifen treatment, whereas models in BIG 1-98 analyses were stratified for randomization option, treatment allocation and age at randomization. Univariate analyses of the Odense cohort were used to assess thresholds for Bcl-2 and ER classified by hierarchical indicator variables [Citation16]. Multistep backward selection was used to remove non-statistically significant (p >0.05) indicator variables from the model. If more than two levels differed significantly from each other, multiple thresholds were allowed. The overall level of statistical significance of variables was assessed by tests of the global hypothesis of no difference among groups. Thresholds were evaluated in multivariate models including the effects of Bcl-2 and ER and adjusted for the effects of HER-2 status, age at surgery, tumor size, axillary nodal status, histological type and tumor grade. In all analyses the stratum with the most observations was chosen as reference. Models were fitted to data by the PROC PHREG of SAS version 9.2 (SAS Institute, Cary, NC, USA). All statistical tests were two-sided, and p-values less than 0.05 were considered statistically significant.
Results
In the Odense cohort of 257 women, the median age at surgery was 61 years with a median duration of 1.8 years of tamoxifen treatment. Thirty-one patients (12%) switched to an aromatase inhibitor. Median potential follow-up for DFS was 10 years after surgery.
Bcl-2 immunostaining was exclusively localized in the cytoplasm. The frequency of cells expressing Bcl-2 protein was high and the intensity of the staining strong in most of the tumors. At least 50% Bcl-2-positive cells were detected in 97% (250/257) of the tumors, a cell-positive frequency from 10–49% was detected in 2% (5/257), and 1–9% positive cells were detected in about 1% (2/257). The intensity of the staining was weak in tumors with 1–9% positive cells; in tumors with 10–49% positive cells the intensity could be either weak, moderate or strong, whereas for tumors with ≥50% positive cells staining was mainly of strong intensity.
Inter-observer agreement was 86.9% and directional differences between observers were not found (p =0.30).
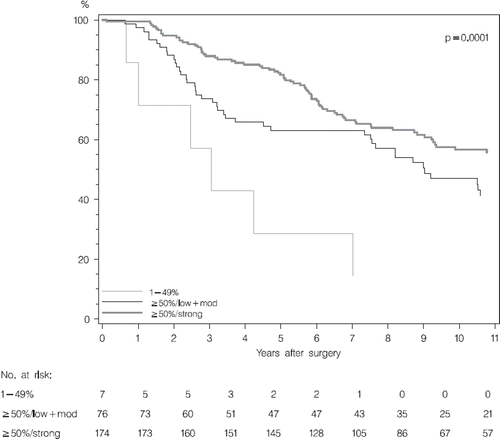
Due to the low frequency of 1–9% scores the classification of Bcl-2 was modified to 1–49%, ≥50%/weak, ≥50%/moderate and ≥50%/strong.
Bcl-2 score was positively associated with ER (p =0.0042), negatively associated with number of positive lymph nodes (p =0.038), histological grade (p <0.0001), and positive HER-2 status (p <0.0001), but not associated with age at surgery or tumor size.
Univariate analysis of the association between Bcl-2 and DFS showed that patients with low Bcl-2 scores of 1–49% had a poor prognosis relative to the reference group of high score ≥50%/strong intensity (). For the group of intermediate score ≥50%/weak and moderate intensity, the prognosis was also worse than the reference group during the first five-year postoperative period ().
Table I. Odense cohort, association between Bcl-2 and DFS and determination of threshold values for Bcl-2 by univariate analysis.a
The Kaplan-Meier curves for the three groups of low, intermediate, and high Bcl-2 score are shown in . In multivariate analysis Bcl-2 was highly significant (p =0.001) whereas this was not the case for ER (p =0.32) (). As in the univariate analysis, a highly increased risk of a DFS event was found in the Bcl-2 low score group relative to the reference group of high Bcl-2 score. In the Bcl-2 intermediate score group, a decreased risk was found 5–10 years after operation.
Table II. Association between Bcl-2 and DFS in the Odense cohort by multivariable analysis.a
In the study population of 1191 patients in the BIG 1-98 study (), the median age at randomization was 61 years. Cross-over to letrozole was done for 132 patients originally allocated to tamoxifen monotherapy. The median potential follow-up for DFS was six years after randomization. The distribution of Bcl-2 score was skewed towards high values with 95.5% of the tumors having ≥50% positive cells; the intensity of the staining within each of the frequency categories was very similar to the results in the Odense cohort.
Table III. Demographic characteristics of Danish patients randomized (n =1396) in the BIG 1-98 study.
Bcl-2 was positively associated with ER (p <0.0001) and negatively associated with HER-2 positive status (p <0.0001) and tumor grade (p <0.0001). Bcl-2 was not associated with age at randomization, histological type, tumor size, or number of positive lymph nodes.
The survival analyses of the BIG 1-98 Danish cohort were based on Bcl-2 thresholds determined in the Odense study supplemented with the preplanned threshold at 10% Bcl-2 positive cells.
The Kaplan-Meier estimates of DFS for the BIG 1-98 Danish Cohort divided in four groups are shown in . In univariate analysis the association between Bcl-2 and DFS was significant (p <0.0002). Patients with the lowest Bcl-2 level (0–9%), and patients with Bcl-2 level of ≥50%/weak and moderate intensity for the first five years, had a poor prognosis relative to the highest Bcl-2 level of ≥50%/strong ().
Figure 2. Kaplan-Meier estimates of DFS according to level of Bcl-2 in Danish Cohort of BIG1-98 (n = 1191).
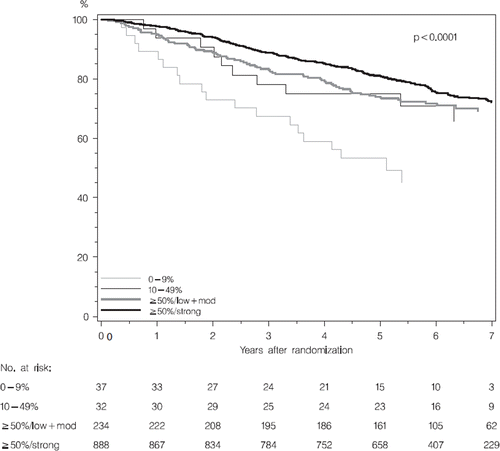
Table IV. Association between Bcl-2 and DFS in the Danish Cohort of BIG 1-98 by univariate analysis.a
The association between Bcl-2 and DFS was also significant (p =0.028) in a multivariable analysis including the classic prognostic variables ER, HER-2, number of positive lymph nodes, tumor size, histological type and grade ().
Table V. Association between Bcl-2 and DFS in the Danish Cohort of BIG 1-98 by multivariable analysis.a
In order to evaluate the value of Bcl-2 in a clinical context of current treatment, we made an additional analysis on a subset of the Danish BIG 1-98 cohort including only patients with normal or unknown HER-2 status (n =1082). Multivariable analysis including non-standard prognostic variables Ki-67 and PgR showed no prognostic effect of Bcl-2 (p =0.06).
Discussion
We evaluated the prognostic effect of Bcl-2 in a specific subgroup of ER-positive tumors from patients treated with endocrine therapy as the only adjuvant medical treatment. Different thresholds of Bcl-2 level and intensity might be required for the different breast subtypes for prognostication; to our knowledge this matter has not been addressed with Bcl-2 in previous studies.
In this study we applied a simple scoring system that assessed both percentage of stained tumor cells and intensity of the staining and demonstrated its reproducibility among two pathologists.
We have hypothesized that Bcl-2 level reflects the activity of the ER and that Bcl-2 will therefore supplement the ER measurement as a prognostic marker in endocrine treated patients. In concordance, a positive correlation between semiquatitative ER and Bcl-2 levels were found. However, we also found some tumors with simultaneous low expression of Bcl-2 and high ER supporting that the presence of large amounts of ER is not identical to an active ER.
We also found a trend towards a quantitative effect of Bcl-2: the patients with the lowest Bcl-2 category having the largest increase in risk, again supporting the idea that Bcl-2 level is a reflection of the activity of the ER and has the ability to predict the prognosis following ER-directed therapy.
We found that low Bcl-2 score correlates with high tumor grade, more positive lymph nodes and high Ki-67, which is in agreement with previous studies showing that Bcl-2 expression is associated with a less aggressive subset of breast carcinomas. We disclosed a strong association between low Bcl-2 score and positive HER-2 status, suggesting that loss of Bcl-2 is associated with activation of alternative growth pathways, which may be contributing to endocrine resistance and therefore lead to a poor outcome for the patient.
We found few tumors with low Bcl-2 score as a natural consequence of the skewed distribution of Bcl-2 expression in these ER-positive populations. Combined with a relatively small sample size of available tumors from the Odense cohort, this limited our ability to explore all relevant thresholds. However, in the larger BIG 1-98 cohort the 10% threshold was applicable and was found to be the only significant threshold for Bcl-2 evaluation. Thus, our data confirm the finding of Dawson et al. [Citation7] that Bcl-2 is an independent prognostic marker in ER-positive patients.
Our finding that Bcl-2 can predict the outcome in an endocrine treated population could be due to poor response to endocrine treatment in tumors with low Bcl-2 because of a less actively functioning ER as hypothesized. The current study lacks an untreated population for comparison since all the patients were treated with endocrine therapy; therefore, only the prognostic but not the predictive value of Bcl-2 could be investigated. Prognostic markers are used to identify high risk patients that will need additional treatment. One of these markers is HER-2. Contemporary patients with HER-2 positive breast cancer will be recommended trastuzumab in combination with chemotherapy. Bcl-2 as a prognostic marker in patients with ER-positive tumors in today's clinical setting is only relevant if they have HER-2 negative status. To evaluate Bcl-2 as a prognosticator in a HER-2 negative population we conducted a subset analysis and no evidence of a significant prognostic impact was observed in the HER-2 negative subset. A possible explanation is the strong correlation between Bcl-2 and HER-2 leaving very few Bcl-2 low tumors when HER-2 positive tumors are excluded.
Bcl-2 IHC is a low cost analysis used in routine diagnostics in pathology departments which makes it attractive for implementation as a marker in breast cancer management. We have confirmed that Bcl-2 can identify a small proportion of patients (3.1%) with negative prognosis independent of other known prognostic markers among patients with ER-positive tumors. However, the clinical relevant subset of patients is those only treated with endocrine therapy that might need additional treatment.
In conclusion, this study shows that Bcl-2 has prognostic significance. However, the use of Bcl-2 as a combined prognostic and predictive marker of DFS is still an open question. Neoadjuvant studies of endocrine treatment with the ability of direct evaluation of treatment response could provide an answer to this question.
Supplementary Table I
Download PDF (160.5 KB)Acknowledgements
The authors thank the steering committee of IBCSG for access to the BIG 1-98 data. We wish to thank “Breast Friends”, “Dansk Kraeftforsknings Fond”, “Danish Agency for Science Technology and Innovation” and “A Race Against Breast Cancer” for financial support. Partial funding for BIG1-98 was provided by the U.S. National Institutes of Health, grant number CA-75362.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- , Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Davies C, Godwin J, Gray R, Clarke M, Cutter D, . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta- analysis of randomised trials. Lancet 2011;378:771–84.
- Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn H-J, . Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol 2009;20:1319–29.
- Schorr K, Li M, Krajewski S, Reed JC, Furth PA. Bcl-2 gene family and related proteins in mammary gland involution and breast cancer. J Mammary Gland Biol Neoplasia 1999;4:153–64.
- Larsen SS, Heiberg I, Lykkesfeldt AE. Anti-oestrogen resistant human breast cancer cell lines are more sensitive towards treatment with the vitamin D analogue EB1089 than parent MCF-7 cells. Br J Cancer 2001;84:686–90.
- Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, . Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res 2006;12:2468–75.
- Cardoso F, Paesmans M, Larsimont D, Durbecq V, Bernard-Marty C, Rouas G, . Potential predictive value of Bcl-2 for response to tamoxifen in the adjuvant setting of node-positive breast cancer. Clin Breast Cancer 2004;5:364–69.
- Dawson SJ, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, . BCL2 in breast cancer: A favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer 2010;103:668–75.
- Gasparini G, Barbareschi M, Doglioni C, Palma PD, Mauri FA, Boracchi P, . Expression of bcl-2 protein predicts efficacy of adjuvant treatments in operable node-positive breast cancer. Clin Cancer Res 1995;1:189–98.
- Kim K, Chie EK, Han W, Noh D-Y, Park IA, Oh D-Y, . Prognostic value of p53 and bcl-2 expression in patients treated with breast conservative therapy. J Korean Med Sci 2010;25:235–9.
- Yang Q, Moran MS, Haffty BG. Bcl-2 expression predicts local relapse for early-stage breast cancer receiving conserving surgery and radiotherapy. Breast Cancer Res Treat 2009;115:343–8.
- Ihemelandu CU, Dewitty RL, Jr., Leffall LD, Jr., Suryanarayana SM, Frederick WA. Clinical significance of p53 and bcl-2 protein coexpression phenotypes in molecular breast cancer subtypes of pre-menopausal and post- menopausal African-American women. Am Surg 2009;75: 776–84.
- Daidone MG, Luisi A, Veneroni S, Benini E, Silvestrini R. Clinical studies of Bcl-2 and treatment benefit in breast cancer patients. Endocr Relat Cancer 1999;6:61–8.
- Moller S, Jensen MB, Ejlertsen B, Bjerre KD, Larsen M, Hansen HB, . The clinical database and the treatment guidelines of the Danish Breast Cancer Cooperative Group (DBCG); its 30-years experience and future promise. Acta Oncol 2008;47:506–24.
- Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thurlimann B, Paridaens R, Smith I, . Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. N Engl J Med 2009;361:766–76.
- Henriksen KL, Rasmussen BB, Lykkesfeldt AE, Moller S, Ejlertsen B, Mouridsen HT. Semi-quantitative scoring of potentially predictive markers for endocrine treatment of breast cancer: A comparison between whole sections and tissue microarrays. J Clin Pathol 2007;60:397–404.
- Walter SD, Feinstein AR, Wells CK. Coding ordinal independent variables in multiple regression analyses. Am J Epidemiol 1987;125:319–23.