Abstract
Introduction. Technological innovations have taken stereotactic body radiotherapy (SBRT) from frame-based strategies to image-guided strategies. In this study, cone beam computed tomography (CBCT) images acquired prior to SBRT of patients with lung tumors was used to study the dosimetric impact of a pure frame-based strategy. Material and methods. Thirty patients with inoperable lung tumors were retrospectively analyzed. All patients had received CBCT-guided SBRT with 3 fractions of 15 Gy to the planning target volume (PTV) margin including immobilization in a stereotactic body frame (SBF). Using the set-up corrections from the co-registration of the CBCT with the planning CT, all individual dose plans were recalculated with an isocenter position equal to the initial set-up position. Dose Volume Histogram (DVH) parameters of the recalculated dose plans were then analyzed. Results. The simulated plans showed that 88% of all fractions resulted in minimum 14.5 Gy to the internal target volume (ITV). For the simulated summed treatment (3 fractions per patient), 83% of the patients would minimum receive the prescription dose (45 Gy) to 100% of the ITV and all except one would receive the prescription dose to more than 90% of the ITV. Conclusions. SBRT including SBF, but without image guidance, results in appropriate dose coverage in most cases, using the current margins. With image guidance, margins for SBRT of lung tumors could possibly be reduced.
Stereotactic body radiation therapy (SBRT) is increasingly used for treatment of non-small cell lung cancer (NSCLC) and metastases in the lung. Several clinical studies have been initiated comparing this treatment modality with conventional fractionated regimes, and promising survival data have been published [Citation1–6]. Different strategies have been chosen regarding treatment equipment and total dose, and dose escalation studies have shown that a high dose delivered in 3–5 fractions is efficient and well tolerated [Citation7–11].
Guidelines for SBRT planning have been described in detail previously [Citation12–15], where a stereotactic body frame (SBF) [Citation13] is recommended for patient immobilization. Following these procedures, Baumann et al. [Citation2] presented progression free survival at three years of 52% in a prospective phase II study of medically inoperable patients with stage I NSCLC.
During the last decade, SBRT of lung tumors has evolved extensively in both dose calculation algorithms and image guidance. The standard dose calculation algorithm in the lung has changed from pencil beam types to more advanced convolution algorithms [Citation16–20]. This change has led to high accuracy in the dosimetry of lung treatment, and the current focus should be directed towards better image guidance.
Cone beam computed tomography (CBCT) gives the opportunity to directly identify the target volume at the day of treatment, and thus to correct the position of the patient according to the planned position [Citation21]. Such image guidance may imply utilizing reduced margins, compared with non-image-guided radiotherapy (IGRT) strategies [Citation22,Citation23]. CBCT guidance and patient immobilization using an SBF, with a highly stable immobilization, is therefore an attractive combination for high-precision SBRT.
The Scandinavian stereotactic radiotherapy study group has, for SBRT of inoperable lung tumors, recommended to prescribe 45 Gy to the PTV margin in 3 fractions. A multicenter randomized phase II study where such SBRT and conventional fractionated radiotherapy are compared has recently finished patient accrual. This protocol; stereotactic precision and conventional radiotherapy evaluation – SPACE [Citation24] – was set up before IGRT was implemented on a large scale. However, during the years of patient inclusion, CBCT at the treatment units have become standard equipment and this could possibly lead to increased precision of treatment delivery to the patients included late in the study. Most importantly, it is not straightforward to assess the impact of geographical misses on patient survival for the sub- cohort that received a traditional frame-based approach without on-line image guidance. However, for patients receiving frame-based CBCT-guided SBRT, the on-line set-up corrections provide a means of identifying patients at risk of underdosage in the absence of image guidance.
In the current work, on-line set-up correction data for 30 patients with inoperable lung tumors receiving frame-based CBCT-guided SBRT was retrospectively collected. Using these correction data, the dosimetric impact of treatment without image guidance was simulated using a treatment planning system. The findings may be important for interpreting results in clinical studies, such as the SPACE trial where a technological development is ongoing during patient inclusion.
Material and methods
Patient selection
In this quality assurance study, data for 30 patients with inoperable lung tumors treated from February 2009 to April 2010 were retrospectively analyzed. All patients were treated with frame-based image-guided SBRT. Patient characteristics are given in .
Table I. Patient and tumor characteristics.
Treatment planning and delivery
Thirty patients with inoperable lung tumors who received SBRT planned and treated according to the guidelines in the SPACE-protocol [Citation24] were analyzed. Immobilization was achieved in a SBF (Elekta, Crawley, UK), where abdominal compression was applied if tumor motion over 10 mm was detected using fluoroscopy. Three patients needed abdominal compression. All patients were scanned with an 8-slice LightSpeed CT-scanner (GE Healthcare, Bucks, UK) with slice thickness of 2.5 mm, and a free breathing CT-scan was used for dose calculation. In addition, all patients received a 4D-CT over a restricted area around the tumor, using the same scanner immediately after the planning scan. From the 4D-CT, a maximum intensity projection (MIP) image series was derived, and co-registered with the planning CT. The gross tumor volume (GTV) was delineated both in the planning CT and the MIP series, and the union of these volumes was assumed to cover all tumor locations during treatment. Additionally, a 5 mm margin was added to create the internal target volume (ITV), also including subclinical disease. The planning target volume (PTV) was extended 5 mm uniformly outside the ITV. Some patients (n = 5) had slight adjustments of margins from these guidelines where individual clinical considerations were taken into account at planning.
Dose planning was performed in Oncentra MasterPlan v.3.3SP3 (Nucletron, Veenendaal, The Netherlands), applying a collapsed cone dose calculation algorithm. The treatment plans contained conformal arcs (median of 4 arcs, range 3–5) and/or static beams (median of 5 static beams, range 1–8). The conformity index (V100/VPTV) was aimed to be below 1.4 for all plans. For five plans the conformity index was slightly above 1.4 because the plans had extraordinary sharp dose gradients towards organs at risk. Normalization was 15 Gy/fraction to the minimum dose voxel of the PTV, and the maximum doses were around 22.5 Gy/fraction (median 22.8 Gy). All patients received three treatment fractions. Initial planning data is summarized in , where median values are given.
Table II. Characteristics of the initial dose plans. Median values are given, with ranges in brackets.
Treatment was delivered at an Elekta Synergy linear accelerator (Elekta, Crawley, UK) equipped with an XVI CBCT-system. The patients were set up in a two-step process. First they were positioned according to the SBF, utilizing tattoos and lasers attached to the frame. In the second process the body frame was positioned according to the room lasers and treatment machine. Online patient repositioning according to the tumor location was done by co-registering the volumetric CBCT-scan acquired at each treatment fraction with the planning CT-scan. The registration was performed by manually matching the visual tumor to the drawn contour of the tumor at planning. A physician approved the first match before treatment.
Replanning
Repositioning data from the CBCT-scans were analyzed for all treatment fractions for the 30 patients. To simulate a treatment without on-line image guidance (frame-based positioning only), the center of the dose plan was shifted in the opposite direction of the repositioning location and the plan was subsequently recalculated. This was repeated for all three treatment fractions. Hence, for each of the patients, four resulting dose plans were analyzed; the original plan and three simulated frame-based treatment plans, in total 120 dose plans.
Results
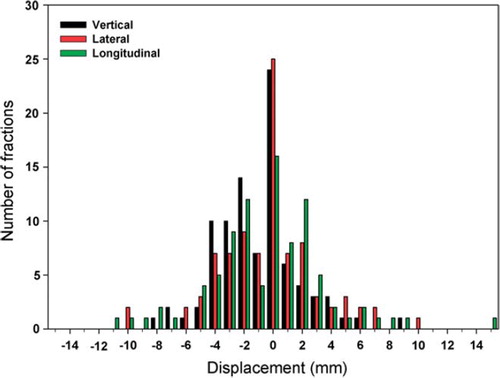
Registration of the CBCT-scans with the planning CT-scans showed an average repositioning vector of 5.2 mm, with mean displacement of −1.0 mm, −0.4 mm and −0.4 mm in vertical, lateral and longitudinal direction, respectively. The systematic (Σ) and random (σ) positioning errors were 2.4 mm and 1.8 mm, respectively, in the vertical direction, 2.7 mm and 2.7 mm in the lateral direction and 3.4 mm and 2.8 mm in the longitudinal direction. Histograms of the registered displacement data from all 90 treatment fractions in the three orthogonal directions are illustrated in .
Figure 1. Registered displacement of the target according to the initial set-up in the SBF compared to the planning CT, as obtained from the CT-CBCT registration. Results in the vertical, lateral and longitudinal directions are displayed for all treatment fractions, 90 in total.
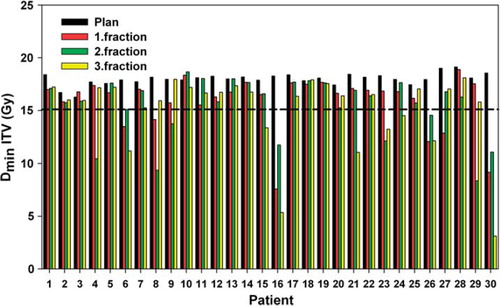
An example of the simulated dose plans in three orthogonal planes is displayed in . The figure illustrates how the dose distribution is shifted according to the CT-CBCT registrations. The calculated minimum dose to the ITV for all 120 plans is displayed in . Of the 90 simulated treatment fractions, assuming frame-based positioning with no image guidance, 69 fractions (76.7%) had a minimum ITV dose of at least 15 Gy, 79 fractions (87.8%) had a minimum of 14.5 Gy and 85 fractions (94.4%) had a minimum of 13 Gy. The average reduction of the ITV minimum dose was 13.8% with a median reduction of 7.5%. Additionally, from the summed plans for each patient, 25 patients (83.3%) would get at least 45 Gy to the ITV, and all patients except one would get 45 Gy to more than 90% of the ITV.
Figure 2. Example of the four plans for a patient. The target volumes appear as solid lines in the axial view and as dotted lines in the reconstructed sagittal and coronal views. Dose isoshades are also displayed in the slices, where the yellow isoshade is the prescription dose (15 Gy).
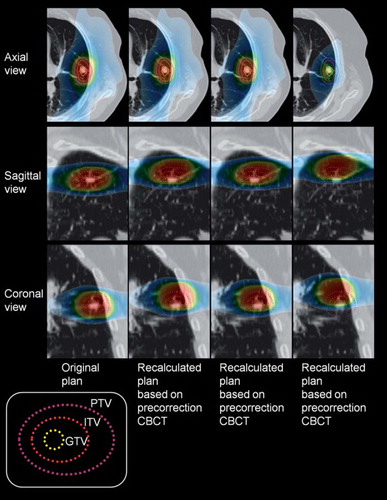
Figure 3. Minimum doses to the ITV for all four plans for all patients. The 15 Gy level indicates the prescription dose.
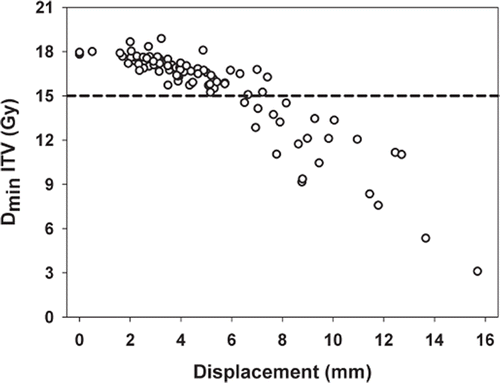
shows the minimum dose to the ITV in all recalculated plans as a function of the displacement vector following the CT-CBCT co-registration. The figure shows that for a displacement below 6–7 mm, the prescription dose is delivered to the ITV, while the minimum dose drops rapidly for larger displacements.
Figure 4. The ITV minimum dose as a function of the absolute displacement vector. Results for all treatment fractions (n = 90) are displayed, where three fractions are summed for each patient (N = 30).
For the spinal cord, no overall change in mean dose was observed over the patient cohort, but for individual patients the change ranged from a 32% reduction to a 20% increase.
Discussion
In this study we have elucidated the dosimetric impact of frame-based SBRT of lung tumors, if no image guidance is available. The results show that the conventional margins applied for patients positioned in the SBF are sufficient for most patients.
In a recent study, Galerani et al. 2010 [Citation25] used CBCT to study the dosimetric impact of online correction for stereotactic lung radiotherapy. Their analysis combined uncertainties in the planning procedure, immobilization and clinical changes of the target volume. The results from that work show a mean dose discrepancy of 26.1% and 23.5% in CTV D99% and CTV D95%, respectively. Their CTV is comparable to the ITV in the present study, but the methodology used is different preventing a direct comparison.
Grills et al. 2008 [Citation26] analyzed set-up errors based on immobilization in an SBF (18 patients) or alpha cradle (six patients) and compared it with image-guided set-up. They found almost identical systematic (Σ) and random (σ) positioning errors for SBF set-up as found in the present work. In addition, they looked into margins needed for frame-based set-up and image-guided set-up, and dose reduction of normal tissue going from a frame-based to an image-based set-up. In the current study we have chosen to focus on the true dose coverage for patient treated with conventional margins and a pure frame-based set-up, as this patient group will be included in the same patient demography as patients with image-guided set-up and comparable margins in, e.g. the SPACE study.
The ITV used in this work was delineated in MIP-series derived from 4D-CT and included the visible tumor with margins for subclinical disease. Conventional margins were applied from the ITV to the PTV. Differences between PTVs generated from 3D- and 4D-CT are expected [Citation27,Citation28], and the differences will have a dosimetric impact. Also, there are potential differences between identifying the ITV from a slow 3D-CT and a 4D-CT [Citation29], and free breathing CBCT does not necessarily illustrate the same ITV as found from a MIP-projection from a 4D-CT [Citation30]. These discrepancies should also be taken into account when considering ITV-PTV margins. Furthermore, there are indications that conventional margins accounting for subclinical disease, at least for NSCLC, are underestimated and should be as large as 9 mm [Citation31]. In this work, a margin of 5 mm was applied for subclinical disease and additionally 5 mm from ITV to PTV. However, applying margins to the volume defined from the MIP-projection is a rather conservative approach, as margins are added to all possible locations of the tumor.
One of the patients (patient 30) had an extremely large initial set-up displacement, and the simulated treatment gave only 66% of the ITV the prescription dose of 45 Gy when all three fractions were summed. Checking the set-up documentation of this patient revealed some problems with the vacuum-pillow which could possibly explain the large initial displacements. We have chosen to not exclude this patient, as such problems also could occur using a pure frame-based treatment regime. However, in the guidelines followed (where in-room imaging is not included), it is recommended to perform an additional CT-scan before the first treatment. By current practice, this would not qualify as true image guidance. However, since the patients are moved from the CT-scanner in the SBF or repositioned in the SBF before treatment, it is likely that the initial CT-scan would have identified the problems with patient 30, and implementation of further corrections, is high. If this patient was excluded, all patients would get more than 90% of the ITV covered by 45 Gy, and all except two patients (93%) would get more than 97% of the ITV covered by the same prescription dose. Patient 16 also showed significant deviations in initial positioning compared to the plan, but in this case, no probable explanations were found.
Baumann et al. [Citation2] reported good clinical outcome for patients receiving SBRT for medically inoperable stage I NSCLC. The patients included in that study were mainly treated according to a frame-based strategy. From the current results it is clear that an image-guided strategy would give higher dose to the ITV when applying conventional margins. The results show that the margins could be reduced with image guidance, but one should also bear in mind that reduction of margins should not compromise the coverage of subclinical disease and possibilities for intra-fractional motion. Results by Grills et al. 2008 [Citation26], and unpublished clinical in-house data, indicate that 1–3 mm intra-fractional movements of the ITV gravity center are possible, which are important to take into account when discussing margin reduction.
A frame-based strategy is based on the rigid correlation between the frame and the target volume. However, bony anatomy would be much easier to position accurately relative to the frame than volumes located in soft tissue. kV CBCT applied for lung tumors has the great advantage of visualizing the tumor, and provides the opportunity for direct localization of the target volume. Compared to other treatment sites, this is a great advantage of image guidance in SBRT of lung tumors, and is the largest contraindication of using pure frame-based strategies. In addition, 5.6% of the fractions would give below 13 Gy to the ITV without image guidance, and the ability to identify these potential outliers is also an advantage of image guidance.
It is important to be aware that clinical studies, like the multicenter SPACE study, where the technology has developed significantly while patients have been included, could have an inhomogeneous patient population with respect to tumor dose coverage. This study highlights the dosimetric impact of using a pure frame-based treatment schedule compared to a pure on-line image-guided schedule, which is specifically relevant for the SPACE study. Previously published studies [Citation2] have presented satisfactory outcome data for the frame-based procedure where the SBF was applied. The results of a study with a group of patients with image-guided set-up (but using the same margins as frame-based set-up) would be expected to yield further improved survival. However, improved set-up techniques should be followed by margin reduction and hence less irradiation of normal tissue.
In conclusion, a pure frame-based strategy was demonstrated to be sufficient for covering the ITV by the prescription dose when applying conventional margins for most patients. On-line CBCT image guidance, where the tumor itself is used for guidance, is expected to result in a reduction in the ITV-PTV margins. However, knowledge about uncertainties in the identification of ITV, positioning of the ITV at treatment and intra-fractional movement of the ITV should be gathered to further quantify the margins needed for SBRT of lung tumors.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, . Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys 2010;37:4078–101.
- Baumann P, Nyman J, Hoyer M, Wennberg B, Gagliardi G, Lax I, . Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290–6.
- Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, . Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070–6.
- Fakiris AJ, McGarry RC, Yiannoutsos CT, Papiez L, Williams M, Henderson MA, . Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys 2009;75:677–82.
- McGarry RC, Papiez L, Williams M, Whitford T, Timmerman RD. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: Phase I study. Int J Radiat Oncol Biol Phys 2005;63:1010–5.
- Nagata Y, Takayama K, Matsuo Y, Norihisa Y, Mizowaki T, Sakamoto T, . Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys 2005;63:1427–31.
- Martin A, Gaya A. Stereotactic body radiotherapy: A review. Clin Oncol 2010;22:157–72.
- Timmerman R, Papiez L, McGarry R, Likes L, DesRosiers C, Frost S, . Extracranial stereotactic radioablation – Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest 2003;124:1946–55.
- Lagerwaard FJ, Haasbeek CJA, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008;70:685–92.
- Haasbeek CJA, Lagerwaard FJ, Antonisse ME, Slotman BJ, Senan S. Stage I nonsmall cell lung cancer in patients aged > = 75 years outcomes after stereotactic radiotherapy. Cancer 2010;116:406–14.
- Lo SS, Fakiris AJ, Chang EL, Mayr NA, Wang JZ, Papiez L, . Stereotactic body radiation therapy: A novel treatment modality. Nature Rev Clin Oncol 2010;7:44–54.
- Baumann P, Nyman J, Lax I, Friesland S, Hoyer M, Rehn Ericsson S, . Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol 2006;45:787–95.
- Lax I, Blomgren H, Naslund I, Svanström R. Stereotaxic radiotherapy of malignancies in the abdomen – methodological aspects. Acta Oncol 1994;33:677–83.
- Lax I, Larsson D, Näslund I, Naslund I. Extracranial stereotactic radiosurgery of localized target. J Radiosurg 1998; 1:135–48.
- Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotaxic high-dose fraction radiation-therapy of extracranial tumors using an accelerator – clinical-experience of the first 31 patients. Acta Oncol 1995;34:861–70.
- Ding GX, Duggan DM, Lu B, Hallahan DE, Cmelak A, Malcolm A, . Impact of inhomogeneity corrections on dose coverage in the treatment of lung cancer using stereotactic body radiation therapy. Med Phys 2007;34:2985–94.
- Franks KN, Purdie TG, Dawson LA, . Incorporating heterogeneity correction and 4DCT in lung stereotactic body radiation therapy (SBRT): The effect on target coverage, organ-at-risk doses, and dose conformity. Med Dosim 2010;35:101–7.
- Xiao Y, Papiez L, Paulus R, Timmerman R, Straube WL, Bosch WR, . Dosimetric evaluation of heterogeneity corrections for Rtog 0236: Stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;73:1235–42.
- Pearson M, Atherton P, McMenemin R, McDonald F, Mazdai G, Mulvenna P, . The implementation of an advanced treatment planning algorithm in the treatment of lung cancer with conventional radiotherapy. Clin Oncol 2009;21:168–74.
- Aarup LR, Nahum AE, Zacharatou C, Juhler-Nøttrup T, Knöös T, Nyström H, . The effect of different lung densities on the accuracy of various radiotherapy dose calculation methods: Implications for tumour coverage. Radiother Oncol 2009;91:405–14.
- Korreman S, Rasch C, McNair H, Dirk Verellen D, Uwe Oelfke E, Philippe Maingon F, . The European Society of Therapeutic Radiology and Oncology-European Institute of Radiotherapy (ESTRO-EIR) report on 3D CT-based in-room image guidance systems: A practical and technical review and guide. Radiother Oncol 2010;94:129–44.
- Bissonnette JP, Purdie TG, Higgins JA, Li W, Bezjak A. Cone-beam computed tomographic image guidance for lung cancer radiation therapy. Int J Radiat Oncol Biol Phys 2009;73:927–34.
- Worm ES, Hansen AT, Petersen JB, Muren LP, Præstegaard LH, Høyer M. Inter- and intrafractional localisation errors in cone-beam CT guided stereotactic radiation therapy of tumours in the liver and lung. Acta Oncol 2010;49:1177–83.
- Nyman J. SPACE - Stereotactic precision and conventional radiotherapy evaluation. A multicenter randomized phase II study of stereotactic hypofractionated radiotherapy with body frame versus conventionally fractionated radiotherapy for stage I medically inoperable non-small cell lung cancer 2006. [cited 2011 Sep 9]. Available from: www.nlcg.no/uploads/space_060717.doc
- Galerani AP, Grills I, Hugo G, Kestin L, Mohammed N, Chao KK, . Dosimetric impact of online correction via cone-beam CT-based image guidance for stereotactic lung radiotherapy. Int J Radiat Oncol Biol Phys 2010;78:1571–8.
- Grills IS, Hugo G, Kestfn LL, Galerani AP, Chao KK, Wloch J, . Image-guided radiotherapy via daily online cone-beam CT substantially reduces margin requirements for stereotactic lung radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:1045–56.
- Guckenberger M, Wilbert J, Krieger T, Richter A, Baier K, Meyer J, . Four-dimensional treatment planning for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2007;69:276–85.
- Hof H, Rhein B, Haering P, Kopp-Schneider A, Debus J, Herfarth K. 4D-CT-based target volume definition in stereotactic radiotherapy of lung tumours: Comparison with a conventional technique using individual margins. Radiother Oncol 2009;93:419–23.
- Nakamura M, Narita Y, Matsuo Y, Narabayashi M, Nakata M, Yano S, . Geometrical differences in target volumes between slow CT and 4D CT imaging in stereotactic body radiotherapy for lung tumors in the upper and middle lobe. Med Phys 2008;35:4142–8.
- Vergalasova I, Maurer J, Yin FF. Potential underestimation of the internal target volume (ITV) from free-breathing CBCT. Med Phys 2011;38:4689–99.
- Stroom J, Blaauwgeers H, van Baardwijk A, Boersma L, Lebesque J, Theuws J, . Feasibility of pathology- correlated lung imaging for accurate target definition of lung tumors. Int J Radiat Oncol Biol Phys 2007;69:267–75.