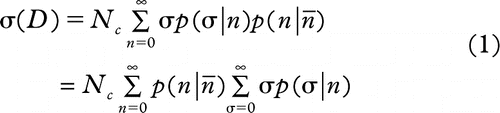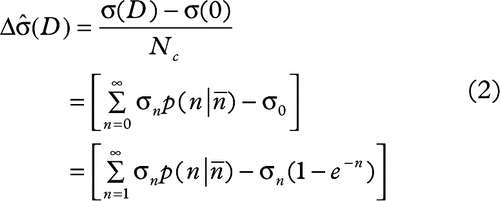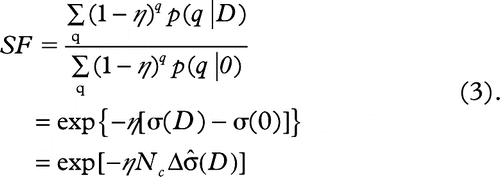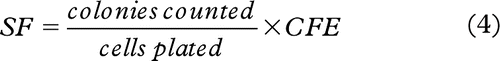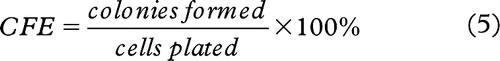Abstract
Introduction. Evidence exists that exposure of non-irradiated cells to Irradiated Cell Conditioned Medium (ICCM) can cause effects similar to those resulting from direct radiation damage. This study attempts to validate the stochastic model, relating absorbed dose to the emission and processing of cell death signals by non-irradiated cells, in vitro in PC3 human prostate cancer cell line. Methods. The recipient cell survival was measured after exposure of cells to ICMM derived from donor cells: a) exposed to radiation doses from 2 Gy to 8 Gy and b) of concentrations varying from 2 × 102 to 6 × 106 irradiated with 2 Gy. Results. Exposure to ICCM, irradiated with doses between 2–8 Gy, resulted in a significant (p < 0.001) decrease in clonogenic survival of non-irradiated recipient cells compared to the control group. However, dose dependency above 2 Gy was not observed, indicating that any dose threshold was below 2 Gy. A significant (p < 0.001) decrease in survival was found in recipient cells exposed to the ICCM, derived from different concentrations of donor cells exposed to 2 Gy, compared to the control group. The recipient cell survival following exposure to ICCM derived from 2 × 102 cells was significantly higher (p < 0.5) compared to the rest of donor cell concentrations, indicating that the toxicity of ICCM depends on the cellular concentration of donor cells. Non-linear regression data fitting provided reasonable agreement with the microdosimetric model for the induction of cell killing through medium-borne signals. Conclusion. For the given cell line and given experimental conditions, significant decreases in cell survival were observed in non-irradiated cells exposed to ICCM derived from donor cells of various concentrations and irradiated with different doses.
It has been established, mostly in low-dose [Citation1], microbeam [Citation2,Citation3], modulated radiation [Citation4] and medium transfer experiments [Citation5], that cells directly hit by radiation may affect cells not damaged by radiation through factors transmitted via extracellular matrix [Citation6] or through gap junctions [Citation7] between cells. Intercellular signals may reach these cells (also known as bystander cells) and produce many of the same effects as direct radiation damage.
Experimental evidence [Citation8,Citation9] indicates that cells directly exposed to low linear energy transfer (LET) radiation release a transmissible cytotoxic factor(s) the into cell growing medium that can be transferred to non-irradiated cells.
It has been demonstrated [Citation10] that exposure to medium from irradiated donor cells, referred to in the literature as Irradiated Cell Conditioned Media (ICCM), may affect recipient cells that are not directly exposed to radiation. Mothersill and co-workers [Citation5] concluded that filtered cell-free medium from irradiated human epithelial cells significantly reduced clonogenic survival and increased the incidence of apoptosis in non-irradiated recipient cells. However, the same research group also demonstrated that cell-to-cell contact through gap junctions had no effect on the ability of medium from irradiated epithelial cell cultures to reduce the clonogenic survival of non-irradiated cells [Citation11]. Similarly designed studies [Citation12] showed that low doses of high-LET α-particles may induce extracellular factors causing excessive cellular transformation, such as sister chromatid exchange (SCE), in unexposed cells. In this experimental work the short-lived SCE-inducing factors were produced in cell-free irradiated culture medium containing serum. The authors concluded that the short-lived transmissible factors could be involved in the production of super-oxide radicals, while the long-lived factors were considered to be cytokines [Citation5]. Mothersill and Seymour suggested [Citation13] that these short-lived radicals may originate from serum components of the culture medium while long-lived messengers might be released by activated donor cells.
It is still unclear which transmissible cytotoxic factors donor cells release into growth medium during radiation exposure. However, several factors and key pathways at the molecular level have been identified [Citation14]. Among them are CycloOXygenase 2 (COX2), Death Receptor 5 (DR5), InterLeukin (IL), Jun N terminal Kinase (JNK), Nitric Oxide (NO) and NO synthase 2 (NO2), Reactive Oxygen Species (ROS), Transforming Growth Factor β (TGβ) and its receptor (TGβR), and Tumour Necrosis Factor α (TNF α).
In this study, we assessed the toxicity of ICCM for the end point related to cell survival. The ability of non-irradiated cells to form colonies after exposure to medium-borne signals released by irradiated cells measures the long-term cell survival. A standard medium transfer colony-forming assay was chosen as it encompasses all types of cellular damage including early apoptosis, mitotic catastrophe (leading to late apoptosis or necrosis), permanent growth arrest followed by necrosis and cell senescence.
Given the variability of the bystander responses reported in the literature in the current work we aimed to determine whether the occurrence and the magnitude of medium-borne signals could be influenced by different radiation doses received by donor cells. We also aimed to investigate whether cell concentration, from which ICCM was collected, has an impact on its toxicity. It was out of the scope of the current work to identify diffusible cytotoxic components released by directly irradiated cells into the extracellular environment.
Material and methods
Microdosimetric model for the emission of medium-borne cell death signals and recipient cell survival probability
Stewart et al. [Citation15] proposed a microdosimetry [Citation16] based model that predicts the magnitude of the emission of death signals released by irradiated donor cells into growth media and that evaluates the probability of recipient non-irradiated cells to survive these signals.
It was assumed that death signal emission is proportional to the number of donor cells releasing these signals due to radiation hits. The secretion of death signals was considered to be a dose related stochastic process depending on the amount of energy deposited into the intra- and extracellular environments.
A brief summary and the main assumptions used in the work of Stewart et al. are as follows:
1. the expected number of death signals released by all hit cells (σ) is expressed by Equation 1:
2. change in the average number of death signals per donor cell ![]() attributed to the radiation dose received is shown in Equation 2:
attributed to the radiation dose received is shown in Equation 2:
3. the survival fraction (SF) of the recipient cells exposed to ICCM containing σ death signals is expressed by Equation 3:
The parameters used in these equations and their meanings are defined as in .
Table I. The parameters used by Stewart et al. in the microdosimetry based model predicting cell-killing through medium-borne death signals.
According to Equation 3, the fraction of non- irradiated recipient cells, surviving transfer into the ICCM, decreases exponentially with increase in the signal intensity ![]() or the number of irradiated donor cells (Nc).
or the number of irradiated donor cells (Nc).
In the current work we aimed to investigate whether the toxicity of ICCM depends on the absorbed dose received by donor cells and on the cellular concentration of donor cells releasing toxic medium-borne signals due to radiation hits.
Cell culture
The PC3 human prostate epithelial adenocarcinoma cell line, initially established from a patient’ bone marrow metastasis [Citation17] was used in this work. This cell line was kindly provided by Professor Wayne Tilley from the Dame Roma Mitchell Cancer Research Laboratories, University of Adelaide and the Hanson Institute.
The PC3 cell line was used as the donor, from which the ICCM was derived. The same cell line was used as the recipient non-irradiated cells, to which the ICCM was transferred.
These cells were cultured as a monolayer in RPMI 1640 (Sigma, Sigma-Aldrich Co, USA) growth medium, supplemented with 10% foetal calf serum (FCS) (Gibco, USA), 1 mM L-Glutamine, 1% sodium pyruvate, 1% ascorbate, 1% penicillin/ streptomycin at 37oC in a humidified atmosphere containing 5%/CO2.
Cell irradiation
Cell irradiation was performed by using a 6 MV x-ray beam produced by a Varian linear accelerator (Varian, Palo Alto, CA, USA) at the Royal Adelaide Hospital Radiation Oncology Department (South Australia).
Irradiation was performed at 100 cm from the beam focal spot with a 20 × 20 cm2 radiation field size by applying 3 Gy/min clinically used nominal dose rate. The culture flasks were placed on top of 1.5 cm thick solid water build up sheets to achieve electronic equilibrium at the cell surface. To avoid irradiation through air gaps cells were treated posteriorly with the gantry positioned at 180o. To ensure full scatter conditions a custom made wax phantom was applied in conjunction with a 5 cm thick solid water slab to cover the flasks.
Cells were irradiated in T-75 flasks (Corning, USA), containing 15 ml of the culture medium per flask.
To investigate the toxicity of the ICCM, based on the dose received by donor cells 2, 4, 6 and 8 Gy absorbed dose, were delivered to each flask to the cell monolayer surface.
To investigate the toxicity of the ICCM based on the donor cells concentration 2 Gy absorbed dose was delivered to each flask containing different amounts of cells.
The doses, which closely mimic human exposure scenario in radiation therapy, were chosen in this study.
The beam-on-time parameters, so called monitor units (MU), were determined by using LiF100 thermoluminecent chip dosimeters (TLD). TLDs were used to establish the MUs required giving the stated absorbed dose to the cell applying the described radiation setup.
Prior to irradiation the machine radiation output was routinely checked with a Daily QA 3™ device (Sun Nuclear, USA) ensuring that 1 Gy of absorbed dose was delivered under reference conditions.
Following irradiation the culture flasks were placed into the incubator immediately.
ICCM
I. Based on the absorbed dose received by donor cells
The medium transfer protocol used to derive the ICCM can be found described in detail elsewhere [Citation18]. Briefly, approximately 1.3 × 106 of PC3 donor cells per T75 flask were plated 24 h prior to irradiation to let the cells adhere to the flasks surface. Then, 15 ml of the fresh cell culture medium was added to each flask just before irradiation.
Absorbed doses of 2, 4, 6 and 8 Gy were delivered to each flask using the linac irradiation setup described above. The control flask, containing the same amount of PC3 donor cells received no irradiation.
The ICCM from donor cells, corresponding to each dose received, was carefully collected one hour post irradiation and filtered through a 0.22 μm filter to ensure that no cells or other debris were left in the medium.
One hour incubation for the generation of ICCM was chosen based on published work that demonstrated no change in the recipient cells responses when the medium was harvested over the period from 30 minutes up to 24 hours [Citation5].
II. Based on the cellular concentration of donor cells exposed to the same dose
Donor cells corresponding to the approximate cellular concentrations of 2 × 102, 5 × 103, 5 × 104, 5 × 105 and 5 × 106 per T75 flask were seeded 24 hours before irradiation.
Each flask contained 15 ml of the fresh culture medium and was exposed to an absorbed dose of 2 Gy. A T75 flask containing cell-free culture medium was used as the control.
Irradiated cell condition medium from the donor flask, corresponding to each cellular concentration, was carefully harvested one hour after irradiation and filtered through a 0.22 μm filter to ensure that no cells or other debris were still present in the medium. Cell-free culture medium from the control flask was collected at the same time.
Clonogenic assay
Determination of cell survival after direct irradiation of cells or exposure to ICCM was performed by the clonogenic assay technique of Puck and Marcus [Citation19].
Cellular survival of directly irradiated donor cells
Following the ICCM collection from the donor flasks exposed to different amounts of radiation dose, cells were washed with Hanks balanced salt solution (HBSS) and harvested from flasks [i.e. released by incubation with trypsin (a 1:1 solution of 0.25% trypsin and 1 mM EDTA] for five minutes in 37°C at 5% CO2). Cells were resuspended in HBSS, containing 5% FCS and centrifuged (4000 rpm, 4°C). Following detachment, the cells were washed once. Cells’ pellets were resuspended in a fresh growth medium, and syringed carefully to produce a single cell suspension.
The number of viable cells was determined by using trypan blue exclusion. An aliquot of the cell suspension was mixed with trypan blue dye and counted using a hemocytometer.
Cells were plated into six well dishes (Corning, USA) containing 2 ml of the fresh culture medium. The plating density was chosen based on the preliminary investigation of the PC3 cloning efficiency and the radiation doses received. The seeding densities varied from 200 cells/well for the control group up to 3300 cells/well for the flask receiving 8 Gy.
Survival of the recipient cells receiving the ICCM derived from the donor cells exposed to varying radiation doses
Two hundred non-irradiated PC3 cells, called the recipient cells, were plated in each well into six well dishes (Corning, USA) at the same time as the donor cells and incubated in 37°C at 5% CO2 for 24 hours.
Each well contained 2 ml of the culture media which was replaced with 2 ml of the ICCM collected from the donor cells received 2, 4, 6 and 8 Gy of the absorbed dose or non-irradiated.
Survival of the recipient cells exposed to the ICCM derived from various concentrations of the donor cells
Two hundred PC3 recipient cells were plated per well into six well dishes (Corning, USA) at the same time as the donor cells and incubated in 37°C at 5% CO2 for 24 hours.
Each well received 2 ml of the ICCM derived from the 2 × 102, 5 × 103, 5 × 104, 5 × 105 and 5 × 106 of donor cells exposed to 2 Gy absorbed dose. The control group received cell-free medium exposed to 2 Gy.
For both experiments all culture dishes were incubated for two weeks according to the previously estimated PC3 doubling time in order to mature into colonies containing more than 50 cells per colony and to exclude cells which have a limited growth potential.
Colonies were then fixed with methanol, stained with crystal violet and counted using a stereomicroscope.
The colony forming ability of cells receiving either direct radiation damage or being exposed indirectly to the ICCM was based on the counted number of colonies. Cell survival fractions for each treatment were estimated by applying the following ratio [Citation20]:
Where CFE is the colony forming efficiency estimated from the non-irradiated control group by applying the following ratio:
The number of surviving colonies in control plates, scored for each experimental setup, was considered as 100%. The colonies estimated from the treated samples were normalised to the controls. In each experiment cell survival was estimated by using six-fold samplings.
Statistical analysis
Dose response of cell survival data measured from six-fold samplings was expressed as means ± SEM. The level of significance between treated and control groups was analysed in Graph Pad Prism 5 software (GraphPad Software, Inc., CA, USA) with one way ANOVA analysis of variances, the test which determines how a response was affected by one factor, followed by Tukey's Multiple Comparison Test. This statistical approach is different from a regular t-test as it considers the scatter of all groups, giving the test more power to detect differences. Furthermore, multiple comparisons allow evaluation of the significance level considering the entire family of comparisons instead of each comparison individually. P-values of less than 0.05 were considered to be statistically significant. (In Graph Pad Prism 5 software the level of statistical significance can be chosen between 0.05, 0.01, or 0.001, which graphically presented as *, ** and *** symbols respectively). Cell survival curves were fitted in the Graph Pad Prism 5 software by applying non-linear regression one phase decay model.
Results and discussion
The experiment reported here was designed to investigate whether survival of non-irradiated cells exposed to the ICCM depends on the amount of radiation absorbed by donor cells from which the medium was harvested. The toxicity of the ICCM was also analysed based on the concentration of donor cells. The results and discussion are presented in the following sections.
I. Survival of cells received the ICCM derived from donor cells exposed to varying radiation doses
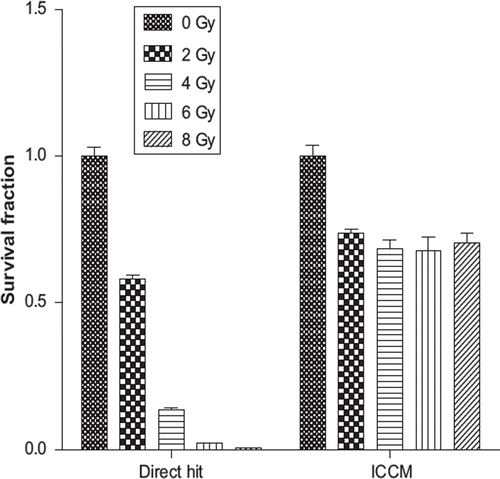
There were significant (p < 0.001) decreases in cellular survival of the recipient cells that received ICCM derived from the donor cells exposed to 2, 4, 6 and 8 Gy absorbed radiation doses compared to the control group (see ).
Figure 1. The survival fraction of non-irradiated PC 3 cell after receiving ICCM from donor cells exposed to 0, 2, 4, 6 and 8 Gy of absorbed dose.
In the current work it was observed that exposure of PC3 recipient cells to the ICCM, containing the currently unknown medium-borne signals, caused changes in colony forming abilities in this cell line.
However, it can be noted that the media harvested from each irradiated group of the donor cells was approximately equally toxic regardless of the radiation dose. According to there were not any significant dose-dependant differences observed between survival of cells exposed to ICCM treated with 2, 4, 6 or 8 Gy radiation doses.
Table II. One-way analysis of variances with Post ANOVA Tukey’s Multiple comparison tests of the PC3 cell survival after receiving ICCM exposed to 0, 2, 4, 6 and 8 Gy radiation doses.
This was in contrast to the survival of cells that received direct radiation damage (see ), which indicates rapid, dose-dependant decrease in SF compared to the cells receiving indirect radiation damage.
The difference in cell survival patterns between direct and indirect radiation damages suggests that perhaps donor cells, activated by relatively high radiation doses, released a similar amount of transmissible cytotoxic factor(s) into the cell growing medium regardless of the doses received.
It appears that initially emitted death signals may increase with increasing number of hits received by donor cells. However, it saturates when the number of hits per cell reaches a certain value [Citation21].
There was rapid drop-off (26.6%) in the cell survival when the recipient cells received ICCM exposed to 2 Gy dose. Nevertheless, after this dose point the fractions of surviving cells exposed to ICCM treated with 4, 6 or 8 Gy dose remained stable (31.5%, 32.2% and 29.7%).
This indicates that the emission of medium-borne signals becomes saturated at a certain level of radiation exposure, which was below 2 Gy.
Our observation is in good agreement with the experimental work of Seymour and Mothersill [Citation18] who observed a relatively constant effect of the ICCM on cell survival in human epithelial cell lines. However, they concluded that the magnitude of the effect appeared to saturate at doses in the range of 0.03–0.05 Gy. Their study reported that for ICCM exposed to doses higher than 0.5 Gy the clonogenic cell deaths observed were either ‘a result of a dose-dependent non-bystander effect or a dose-independent bystander effect’.
Another research group [Citation22] observed no significant difference in ICCM toxicity generated by cells irradiated with either 0.5 or 5 Gy in the experimental work with human keratinocytes. This indicates the saturation of the medium-borne death signals at doses below 0.5 Gy in that experiment.
Nevertheless, Maguire et al. [Citation23] observed in human keratinocytes a significant dose-dependent increase in mitochondrial mass per cell exposed to ICCM irradiated with 5 mGy and 0.5 Gy doses. The reported results suggest that the toxicity of ICCM was dose dependent but only at the low level of radiation exposure below 0.5 Gy.
In the current experimental work we were able to observe significant decrease in the cell survival exposed to the ICCM. The cell survival curve fitting (χ2 = 0.99) confirmed an exponential relationship between the fraction of the cell surviving exposure to ICCM and the absorbed doses received by donor cells (see ).
Figure 3. The exponential fit according to Stewart et al. of PC3 cells survival fraction after exposure to ICCM from donor cells receiving a direct hit of radiation ranging from absorbed doses of 2 to 8 Gy.
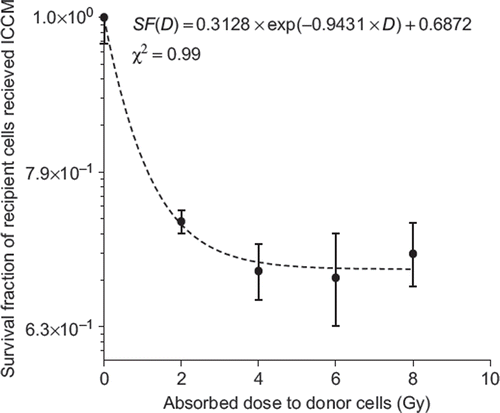
However, we did not observe dose dependant variation in ICCM toxicity above 2 Gy, which was most likely a result of the high level of the radiation exposure used. The secretion of medium-borne signals by PC3 donor cells into the extracellular environment may increase at low doses. It reached a plateau and became saturated after a currently unknown absorbed dose.
To identify a dose threshold and to further analyse a dose-dependent signal emission of the PC3 cells the low dose region (below 1 Gy) needs to be investigated.
II. Survival of the recipient cells receiving ICCM derived from varying concentrations of the donor cells exposed to 2 Gy
In this experiment it was investigated whether the amount of donor cells releasing transmissible cytotoxic factor(s) may affect the toxicity of ICCM.
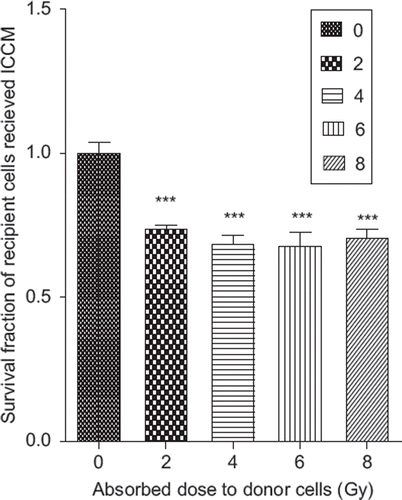
A significant decrease in cell survival, according to the , was found in the recipient cells exposed to the ICCM derived from donor cells of the different concentrations (2 × 102, 5 × 103, 5 × 104, 5 × 105, 1.3 × 106 and 6 × 106) exposed to a 2 Gy radiation dose compared to the control that received cell-free non-irradiated media (see ).
Table III. One-way analysis of variances with Post ANOVA Tukey’s Multiple Comparison Test of the PC3 recipient cells survival exposed to the ICCM derived from varying concentrations of the donor cells exposed to 2 Gy radiation dose.
Figure 4. The survival fraction of recipient PC 3 cells after receiving ICCM exposed to 2 Gy of an absorbed dose derived from donor cells of different cellular concentrations (0, 2.0E + 02, 5.0E + 03, 5.0E + 04, 5.0E + 05, 1.3E + 06, 5.0E + 06) cells per 15 ml. The control group was exposed to cell-free irradiated media received 2 Gy of an absorbed dose.
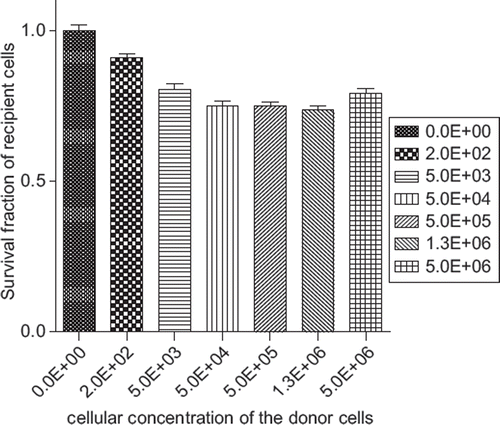
These differences in SFs, presented in the 1–6 comparison tests in , may be attributed to either the amount of radiation received by the ICCM or to the cellular concentration of donor cells releasing medium-borne signals.
However, further evaluation of the rows 7–11 presented in revealed significant differences (p < 0.001) between cells surviving the exposure to the ICCM, derived from 200 donor cells exposed to 2 Gy, and the rest of donor cell concentrations (12–21 comparison tests) receiving the same dose. These results exclude the radiation dependence component in assessing ICCM toxicity and demonstrate the variation among the different cellular concentrations releasing medium-borne signals.
Thus, it was shown that SF of non-irradiated cells was significantly reduced after receiving ICCM harvested from 200 donor cells. However, after the 5000 cell concentration point the toxicity of ICCM reaches a plateau and becomes saturated.
This indicates that for the PC3 cell line under the current experimental conditions, there was an observable decrease in SF of non-irradiated cells exposed to ICCM depending on the cellular concentration.
Observed literature revealed no data of similar design to investigate ICCM toxicity based on the cellular concentration of donor cells. However Ryan et al. [Citation24] investigated the intensity of medium-borne signals based on the dilution of ICCM derived from the six human cell lines which were transferred to the common reporter HPV-G recipient cells. Their study revealed that ICCM from cells of four cell lines induced a bystander effect in HPV-G reporter cells, confirming that signal production was an essential factor. This experimental work also confirmed that the intensity of ICCM varied with dilutions.
Ryan et al. suggested that cell survival would decrease linearly until a plateau was reached and the bystander effect was abolished. It was considered that the effect of ICCM from the different cell concentrations reached a plateau at different dilutions. It was also suggested that the intensity of the emission of medium-borne signals was correlated with the cell line’ radiosensitivity.
Our cell survival curve fitting (χ2 = 0.9) revealed an exponential relationship between the fraction of cells surviving the exposure to the ICCM and the cellular concentration of donor cells releasing medium-borne signals (see ). It appeared that the toxicity of ICCM increased exponentially with donor cell concentration, reached plateau and become saturated after the 5000 cells concentration point.
Figure 5. The exponential fit of PC3 cells survival fraction after exposure to the ICCM derived from 0.2 × 102, 5 × 103, 5 × 104, 5 × 105, 1.3 × 106 and 6 × 106 donor cells per 15 ml exposed to an absorbed dose of 2 Gy.
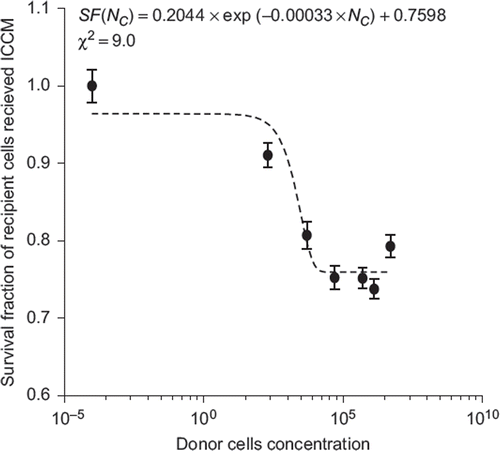
In the current experimental work it was investigated whether the fraction of non-irradiated recipient cells, surviving the exposure to ICCM, was dependent on the cellular concentration and the absorbed dose received by donor cells.
It was assumed that the SF of the recipient cells exposed to ICCM exponentially decreases (as expressed in Equation 6) with increase in the signal intensity [In line mathe (D)] and the number of irradiated donor cells (Nc) emitting medium-borne death signals.
The data measured in the current work were fitted with Equations 7 and 8.
These equations provide a reasonable agreement with Equation 6 proposed by Stewart.
Thus, an Equation 7 indicates the exponential decrease in recipient cell survival as a function of the doses absorbed by donor cells. Additionally, Equation 8 demonstrates that the intensity of the ICCM, affecting recipient cells, was dependent on the concentration of donor cells releasing toxic medium-borne signals as a result of radiation hit.
It was assumed that the constants presented in the Equations 7 and 8 depended on experimental setup, cell growing and treatment conditions.
Similarly to direct radiation damage intercellular-mediated effects may depend on many factors related to biological and exposure conditions presented in a certain experimental reference frame.
Thus it has been demonstrated that the magnitude of the intercellular-mediated effects was dependent on the serotonin level present in FCS [Citation25].
The choice of relevant experimental times in medium transfer experiments was shown [Citation26] to be crucial not only for identifying the effects but also for detecting them. Still, the previously conducted study by Mothersill et al. [Citation5] demonstrated insignificant variations in non-irradiated cell responsiveness to ICCM derived either 30 minutes or 24 hours after radiation exposure.
Additionally, it was shown that radiation induced intercellular-mediated damage was cell line dependant [Citation5,Citation27]. However, another research group demonstrated that it did not correlate with cellular intrinsic radio sensitivity [Citation28].
Dose and dose rate dependence of the medium transfer effect is another controversial point. Several studies suggest that dose [Citation18,Citation22,Citation28] and dose rate [Citation29] are uncorrelated in their medium transfer experiments. However, another research group [Citation23] confirmed the opposite dose dependant relationship.
Conclusion
The microdosimetry based stochastic model of Stewart et al. predicts the magnitude of the emission of death signals released by irradiated donor cells into growing media and evaluates the probability of recipient non-irradiated cells to survive these signals. It provides a more systematic approach to analyse dose dependency and signal potency in medium transfer experiments. In the current work we made an attempt to validate the proposed formalism of Stewart et al. which predicts an exponential decrease in recipient cell survival based on the energy deposition to donor cells and the number of donor cells releasing these signals due to radiation hit. It was not the intention of this work to determine the mechanisms underlying the emission of death signals.
Significant reduction in PC3 cell survival after receiving ICCM was observed. Data fitting revealed an exponential decrease in recipient cell survival; however, it was not possible to identify a dose threshold due to the saturation of the effect at a currently unknown dose. This can be attributed to either saturation in signal generation due to limited signal potency or saturation in recipient cell responses. It appeared that death signal emission may increase with increasing number of radiation hits to a certain target and with increasing number of targets able to emit death signals. However, the effect saturates when it reaches a specific value in a number of hits or in an amount of critical targets.
Additional data, preferably performed in a more consistent experimental reference frame, are needed to determine the origin of the critical target(s). In this study we confirm that the stochastic microdosimetry based model of Stewart et al. captures the main trends observed in low-LET medium transfer effects.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Nagasawa H, Little B. Induction of sister chromatid exchanges by extremely low doses of alpha particles. Cancer Res 1992;52:6394–6.
- Hei TK, Wu LJ, Liu SX, Vannais D, Waldren CA, Randers-Pehrson G. Mutagenic effects of a single and an exact number of alpha particles in mammalian cells. Proc Natl Acad Sci USA. 1997;94:3765–70.
- Prise KM, Belyakov OV, Folkard M, Michael BD. Studies of bystander effects in human fibroblasts using a charged particle microbeam. Int J Radiat Biol 1998;74:793–8.
- Mackonis EC, Suchowerska N, Zhang M, Ebert M, McKenzie DR, Jackson M. Cellular response to modulated radiation fields. Phys Med Biol 2007;52:5469–82.
- Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol 1997;71:421–7.
- Mothesill K, Seymour, C. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: Evidence for release during irradiation of a signal controlling survival into the medium. Rad Res 1998;149:256–62.
- Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha-particle irradiated to nonirradiated cells. Proc Natl Acad Sci USA 2001;98:473–8.
- Shao C, Aoki M, Furusawa Y. Medium-mediated bystander effects on HSG cells co-cultivated with cells irradiated by X-rays or a 290 MeV/u carbon beam. J Radiat Res (Tokyo) 2001;42:305–16.
- Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat Prot Dosimetry 2002;99:169–72.
- Mothersill C, Rea D, Wright EG, Lorimore SA, Murphy D, Seymour CB, . Individual variation in the production of a ‘bystander signal’ following irradiation of primary cultures of normal human urothelium. Carcinogenesis 2001;22:1465–71.
- Mothersill C, Seymour CB. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: Evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res 1998;149:256–62.
- Lehnert BE, Goodwin EH, Deshpande A. Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res 1997;57:2164–71.
- Mothersill C, Seymour C. Radiation-induced bystander effects: Past history and future directions. Radiat Res 2001;155:759–67.
- Prise K, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 2009;9:351–60. Epub 2009 Apr 20.
- Stewart RD, Ratnayake RK, Jennings K. Microdosimetric model for the induction of cell killing through medium-borne signals. Radiat Res 2006;165:460–9.
- Microdosimetry. ICRU report. 1983;36.
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979;17: 16–23.
- Seymour CB, Mothersill C. Relative contribution of bystander and targeted cell killing to the low-dose region of the radiation dose-response curve. Radiat Res 2000;153:508–11.
- Puck TT, Marcus PI. Action of x-rays on mammalian cells. J Exp Med 1956;103:653–66.
- Hall ED, editor. Radiobiology for the radiologist. Maryland, USA: Harper & Row; 1978.
- Liu Z, Prestwich WV, Stewart RD, Byun SH, Mothersill CE, McNeill FE, . Effective target size for the induction of bystander effects in medium transfer experiments. Radiat Res 2007;168:627–30.
- Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br J Cancer 2000;83:1223–30.
- Maguire P, Mothersill C, Seymour C, Lyng FM. Medium from irradiated cells induces dose-dependent mitochondrial changes and BCL2 responses in unirradiated human keratinocytes. Radiat Res 2005;163:384–90.
- Ryan LA, Smith RW, Seymour CB, Mothersill CE. Dilution of irradiated cell conditioned medium and the bystander effect. Radiat Res 2008;169:188–96.
- Mothersill C SR, Smith RW, Singh H, Seymour CB. Serum serotonin levels determine the magnitude and type of bystander effects in medium transfer experiments. Radiat Res 2010;174:119–23.
- Balduzzi M, Sapora O, Matteucci A, Paradisi S. Modulation of the bystander effects induced by soluble factors in HaCaT cells by different exposure strategies. Radiat Res 2010; 173:779–88.
- Vines AM, Lyng FM, McClean B, Seymour C, Mothersill CE. Bystander signal production and response are independent processes which are cell line dependent. Int J Radiat Biol 2008;84:83–90.
- Howe O, O’Sullivan J, Nolan B, Vaughan J, Gorman S, Clarke C, . Do radiation-induced bystander effects correlate to the intrinsic radiosensitivity of individuals and have clinical significance? Radiat Res 2009;171: 521–9.
- Gow MD, Seymour CB, Byun SH, Mothersill CE. Effect of dose rate on the radiation-induced bystander response. Phys Med Biol 2008;53:119–32.