Abstract
Background. Cutaneous recurrences of breast cancer may cause considerable discomfort due to ulceration, oozing, and pain and can also be difficult to treat. Electrochemotherapy is a localised anticancer treatment using electric pulses to make cell membranes permeable, augmenting uptake of chemotherapeutic drugs, and thus enabling highly efficient tumour cell kill. This is the first systematic investigation of electrochemotherapy for larger cutaneous recurrences of breast cancer. Patients and methods. We conducted a phase II trial for patients with cutaneous recurrences where no further treatment options were available. Primary endpoint was objective response evaluated by clinical examination. Secondary endpoints included response evaluated by PET/CT, change in lung diffusion capacity, patient reported symptoms, and distress related to bodily appearance. Treatment consisted of bleomycin injection followed by application of electric pulses. Results. Seventeen heavily pre-treated patients received electrochemotherapy. Twelve patients were evaluable (follow-up > 8 weeks). CT showed four (33%) patients achieving over 50% tumour volume reduction, clinical examination showed one CR and one PR (OR 17%). Symptomatic relief included decreasing exudates, odour, and bleeding. Treatment was well tolerated; the main side effect was post-treatment pain. Conclusion. This first phase II study indicates that electrochemotherapy is a promising treatment alternative for cutaneous recurrences of breast cancer.
The five-year incidence of local recurrence of breast cancer is reported to be between 6% and 23% following mastectomy, and around 6% following breast conserving surgery and radiotherapy [Citation1]. Treatment of local recurrence in a previously irradiated post-mastectomy breast cancer patient remains a clinically challenging problem [Citation2]. Therapeutic options include resection, systemic therapy or additional radiation. Despite these approaches some patients develop progressing local cutaneous recurrence [Citation3]. Local tumour control, regardless of any distant metastases, is important since the presence of an uncontrolled cutaneous recurrence may be highly distressing for the patient.
Electrochemotherapy is a local treatment for cutaneous and subcutaneous tumours. It designates the use of high-voltage pulses to transiently make the cell membrane permeable. This can be used to augment the effect of some types of chemotherapy, by enabling passage over the cell membrane of otherwise non-permeating chemotherapeutic drugs [Citation4,Citation5]. An example of such a chemotherapeutic drug is bleomycin; a large, hydrophilic and charged molecule without a specific cellular uptake mechanism [Citation6]. When direct passage to the cell cytosol is facilitated by electric pulses, the cytotoxic effect of bleomycin is enhanced by a factor 300–700 [Citation6]. Thus, while the patient is receiving a standard intravenous dose of bleomycin or bleomycin is administered directly into the tumour, electrodes can be placed in the tumour and drug entry facilitated. Electrochemotherapy has proven highly effective in palliative treatment of small cutaneous tumours including small cutaneous tumours from breast cancer [Citation7–10], and a case report has shown that the treatment may also work for large cutaneous recurrence of breast cancer [Citation11].
This is to our knowledge the first phase II study to systematically investigate the efficacy of electrochemotherapy in patients with large cutaneous recurrence of breast cancer.
Patients and methods
Study design
This is an investigator initiated phase II study. Primary endpoint was response to electrochemotherapy evaluated by clinical examination according to the Response Evaluation Criteria in Solid Tumours (RECIST) criteria version 1.0 [Citation12]. Response was measured in the treated lesions. Systemic disease, new lesions and non-target lesions were not addressed. Secondary endpoints included safety and toxicity, response evaluation with positron emission tomography (PET)/computed tomography (CT), and evaluation of patients in relation to their appearance using the Derriford Appearance Scale (DAS24) questionnaire [Citation13]. The protocol was approved by the Danish Medicines Agency, the Regional Ethics Committee and the Danish Data Protection Agency. Clinicaltrials.gov identifier: NCT00744653.
Patients were recruited from October 2008 to October 2010. Eligible patients had histologically confirmed breast cancer with cutaneous recurrences larger than 3 cm. Inclusion criteria included: written informed consent, age ≥ 18 years, progressive and/or metastatic disease, no further standard treatment available or patient declining the offered standard treatment (patients receiving endocrine therapy or trastuzumab, vinorelbine, capecitabine or paclitaxel could continue this treatment if no regression in cutaneous metastases, otherwise at least two weeks since last chemotherapy), Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2 and life expectancy of at least three months, coagulation parameters within normal range (medical correction allowed), sexually active patients had to use safe anti-contraceptives during and up to six months after last treatment. Ineligibility criteria included acute lung infection, previous intravenous bleomycin treatment exceeding 200,000 international units (IU)/m2, known hypersensitivity to study drugs, pregnancy or lactation in women (serum HCG performed), or treatment with granulocyte colony stimulating factor or other cytokines.
Electrochemotherapy procedure
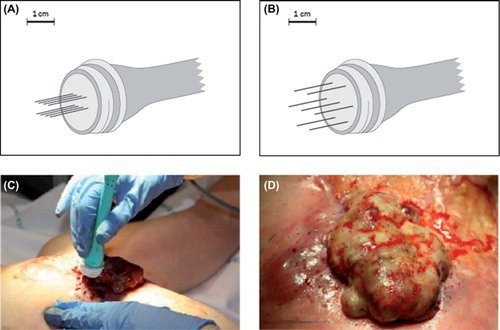
Electrochemotherapy sessions were performed in general anaesthesia based on the standard operating procedures for electrochemotherapy [Citation14]. Bleomycin was primarily administered intravenously (IV) but could also be administered intratumourally. A dose of 15,000 IU/m2 (1000 IU is equivalent to 0.56–0.66 mg of bleomycin) was used for IV administration and 1000 IU/ml was used for intratumoural injection. Electric pulses were applied to the tumour area 8–28 minutes after IV injection of bleomycin or up to 10 minutes after intratumoural injection of bleomycin [Citation14]. Either electrodes with two parallel rows of needles or electrodes with a hexagonal array of needles () were used. Electric pulses (8 pulses of 0.1 ms duration) were delivered using a square wave pulse generator (Cliniporator, IGEA, Carpi, Italy). The applied voltage was 1.0 kV/cm (voltage to electrode distance ratio). The pulses were applied at a frequency of 5 kHz, meaning that each pulse sequence took less than 1 s, and the electrode was moved around to cover the whole tumour lesion (). Small lesions could also be treated but were not registered as target lesions. Patients were offered re-treatment up to three times in cases in which there were areas that had not been sufficiently dealt with in the first treatment session or progression of previously treated lesions was observed.
Figure 1. Electrodes and treatment. Top: Electrode with two parallel rows of needles with 4 mm between the rows and a length of 20 mm (A) and electrode with a hexagonal array of needles with 7.9 mm between the needles with a length of 20 mm or 30 mm. (B). Bottom: Sixty-two-year-old woman with local recurrence of breast cancer on the left chest wall. In (C) the electrode is placed in the tumour, pulses are then delivered and the electrode is moved consecutively to deliver new pulses. In (D) the tumour after treatment is shown, needle marks after the electrode are seen as small red dots.
Assessment of tumour response and safety
Assessment of tumour response was performed by clinical examination (longest diameter of the target lesions), documented by colour photography. At follow-up response was recorded for each target lesion and summed. Response rate was recorded similar to the RECIST criteria [Citation12]. Combined fluorine-18-deoxyglucose PET 60 min post tracer injection and contrast enhanced CT-scan of the thorax was performed with a Gemini PET/CT system (Philips Medical, The Netherlands). On PET scans the changes in standardised uptake value (SUV) were recorded in target lesions. CT-scans were analysed by an onco-radiologist using Eclipse Cone Planning version 8.9 (Varian Medical Systems Inc., California, USA) software with measurement of the largest diameter in the axial plane and three-dimensional (3D) reconstruction of the volume of individual lesions based on manually tracking of the perimeter of the lesions in every slice.
PET image analysis was performed by a specialist in nuclear medicine and an oncologist. Tumour tracking software package (Brilliance Workspace, Philips) was used to measure the maximum SUV within a 3D ellipsoidal region of interest (ROI) covering the target. SUV were automatically drawn by isocontouring in the selected ROI.
Safety was investigated using Common Toxicity Criteria for Adverse Events version 3.0 (CTC) and measurement of diffusion capacity of the lung for carbon monoxide (DLCO) before and three weeks after treatment.
The DAS24 questionnaire addresses aspects of appearance that patients are self-conscious about [Citation13]. Patients were asked to fill out the DAS24 before and 28, 150 and 270 days after treatment.
Statistical considerations
A two stage design based on Simons optimal design for phase II trials was used [Citation15]. A response rate below 20% would not warrant further study. The aim was to reach a response rate greater than 40%. Initially 12 patients were to be treated and followed until evaluation. If fewer than three had partial or complete response at this first stage the study would be terminated, otherwise enrolment would continue until a total of 25 evaluable patients were recruited. As the primary endpoint was response by clinical measurement, this was the basis of decision to stop or continue after 12 patients.
Results
Patient characteristics
Seventeen patients were included and treated, 16 patients were evaluable for toxicity and 12 patients were evaluable for response with a follow-up of at least eight weeks without changes in systemic treatment. Patient characteristics are presented in . Patients presented with a total of 25 large cutaneous recurrences of breast cancer. Smaller recurrences were treated but not included for analysis. Twenty-two tumours were on the chest wall, one tumour on the abdominal wall and two in the supraclavicular region. Fifteen tumours were ulcerated while 10 were infiltrating the skin without ulceration.
Table I. Patients’ characteristics at baseline.
Previous treatment. This protocol was open to patients for whom other treatments had failed. Thirteen patients had previous surgery [breast conserving surgery with subsequent mastectomy (four patients) or primary mastectomy (nine patients); inoperable at diagnosis (four patients)]. All patients had received previous systemic chemotherapy: Cyclophosphamide (nine patients), methotrexate (one patient), epirubicin (12 patients), docetaxel (14 patients), capecitabine (15 patients), vinorelbine (eight patients), gemcitabine (seven patients), paclitaxel (five patients), fluorouracil (three patients), and carboplatin (three patients). Eight patients had received endocrine therapy: Tamoxifen (six patients), letrozole (three patients), fulvestrant (three patients), anastrozole (two patients), exemestan (two patients), and megestrolacetat (two patients). Five patients had received targeted therapies: Trastuzumab (four patients), lapatinib (three patients), and cediranib (one patient). Sixteen patients had received radiotherapy to the chest wall, one patient had not (patient choice).
Follow-up. Five patients exited from the study within eight weeks; two due to other antineoplastic treatment, two had rapid systemic disease progression, and one was lost to follow-up (moved to another part of the country). A total of 12 patients had follow-up of more than eight weeks. Study completion was set at one year, but most patients (11 of 12) withdrew from the study before this due to progressive disease necessitating other treatment (eight patients) or due to deteriorating performance status (three patients).
Treatment procedure
A total of 27 electrochemotherapy sessions were performed (with patients receiving): one treatment (10 patients), two treatments (five patients), three treatments (one patient), or four treatments (one patient), respectively. A second treatment was given in cases where the size of the lesion to be treated required more than one treatment or progression of previously treated lesions was observed. One patient had a third treatment at 162 days after the first treatment and one patient had a third and a fourth treatment at 205 and 289 days after the first treatment. All treatments were performed under general anaesthesia with IV bleomycin in 26 sessions and intratumoural bleomycin in one session (following a specific patient request).
The median time from induction to end of anaesthesia was 85 min (range, 45–125 min). Bleomycin was given as IV infusion over 7 min (range, 3–9 min) and the electrochemotherapy procedure itself from first to last pulse lasted 23 min (range, 10–36 min). A median of 117 pulses (range, 29–173 pulses) were applied in each treatment using a linear needle electrode (two sessions), a hexagonal needle electrode (21 sessions) or both (four sessions).
Response
The median follow-up period was 79 days (range, 11–378 days). Twelve patients had a follow-up for more than eight weeks (median 100 days; range, 56–378 days).
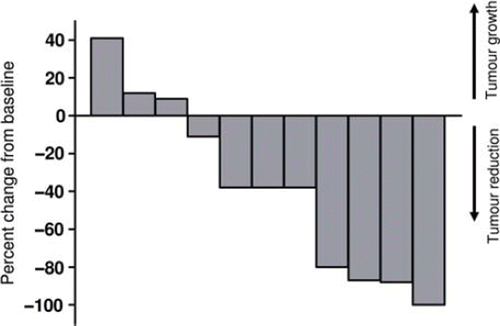
Clinical examination. Through clinical examination a complete response (CR) was observed in one patient and a partial response (PR) in one patient. Stable disease (SD) was observed in nine patients, and progressive disease (PD) in one patient (). As an objective response (CR + PR) was observed in only two patients, the study was terminated after inclusion of 12 evaluable patients. As can be seen below, in and , PET/CT data showed a different picture with higher objective response rate.
Table II. Response evaluation.
Figure 2. Reduction in tumour volume after treatment with electrochemotherapy. Waterfall plot of tumour volume reduction on CT-scan. Twelve patients completed follow-up more than eight weeks, one patient was not evaluable by CT as tumour was indistinguishable from normal skin. Tumour volume of target lesions after one treatment with electrochemotherapy was compared with baseline tumour volume of target lesions and in four patients a reduction of more than 50% was observed.
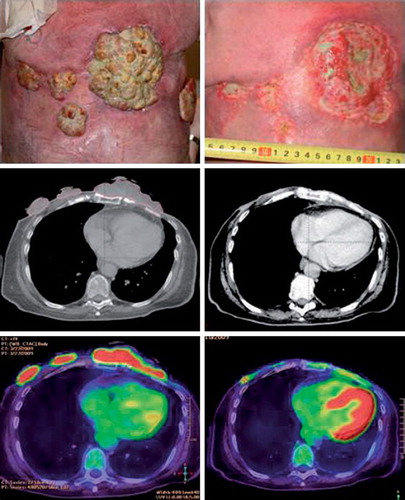
PET/CT. PET/CT was performed median five days (range, 2–15 days) before electrochemotherapy and 24 days (range, 17–59 days) after, three patients did not have post-treatment PET/CT. Responses with CT and PET are listed in . Volume measurement on CT-scan showed a reduction greater than 50% in four patients (of which one had a 100% reduction compared to baseline volume), seven patients had a reduction less than a 50% reduction (of which one had more than a 25% increase compared to baseline volume) and one patient was not evaluable on CT ( and ). An example of a patient's clinical photography, CT and fused PET/CT before and after treatment is shown in .
Figure 3. Patient treated with electrochemotherapy. Sixty-four-year-old woman with loco-regional recurrence of bilateral receptor negative, HER2 – negative breast cancer. Previous treatments over a period of five years included radiotherapy 48 Gy in 24 fractions on both sides and reirradiation with 30 Gy in 10 fractions on the left side, systemic therapy (cyclophosphamide, epirubicin, fluorouracil, docetaxel, gemcitabine, vinorelbine, and capecitabine). Despite all these treatments there was continuous progression of the cutaneous lesions. The column on the left shows image of lesions, CT-scan and PET/CT-scan before treatment scan, column on the right shows image of lesion, CT-scan and PET/CT-scan after two sessions with electrochemotherapy.
Safety and toxicity
Sixteen patients were evaluable for toxicity (). One patient was treated but withdrew before evaluation (the patient moved to another part of the country).
Table III. Toxicity in patients treated with electrochemotherapy.
Treatments were well tolerated with no complications. During anaesthesia patients were ventilated with either an endotracheal tube or a laryngeal mask during anaesthesia. Contractions of muscles on the chest wall or the sternocleidomastoid muscles during pulse application did in some cases cause minor displacement of the laryngeal mask. No changes in electrocardiography were observed during or after pulse application.
Lung function. Fourteen patients had DLCO measured nine days (range, 5–69 days) before and 30 days (range, 21–78 days) after treatment. The median value was 86% (range, 55–144%) of the expected age adjusted value before and 83% (range, 54–156) after treatment, with no significant difference (p = 0.6221 using Wilcoxon signed rank test).
Side effects. Side effects are listed in . CTC grade III pain after treatment was observed in seven (44%) patients. In four patients (25%) the pain was resistant to increased dosage of opioids, lasted up to two months, and was described as neuropathic by some patients. Four patients reported decreased pain after treatment.
CTC grade III infection was observed in one patient (6%). Examination showed a local infection but no signs of a systemic infection. Six patients (38%) reported reduced exudates after treatment, and four patients (25%) reported reduced bleeding ().
Table IV. Patient reported symptoms.
Eight of the 16 patients evaluable for toxicity complained of malodorous smell before treatment due to large ulcerated lesions. Of those five patients reported improvement in odour control after treatment. Two patients reported no change and three patients reported deterioration in odour control lasting up to two months. In the eight patients without odorous tumours before treatment no change was observed after treatment.
Questionnaire
Evaluation of patient self-perception of appearance with the DAS24 questionnaire was a secondary endpoint of this study and completion of the questionnaire was optional. Nine patients completed the DAS24 before and 49 days (range, 28–111 days) after treatment. No significant difference in score was recorded (data not shown).
Discussion
This study shows that electrochemotherapy may lead to symptomatic and objective responses in heavily pre-treated patients with cutaneous recurrence of breast cancer. Even though considerable progress in the treatment of breast cancer has been achieved over the years, relentlessly progressing cutaneous recurrences remains a challenge for the treating physician as well as a significant cause of patient morbidity and distress. The patients seen in this study had extensive previous treatments including surgery, radiotherapy, and several regimes of systemic treatment. Despite this, objective responses were obtained with electrochemotherapy. There are several explanatory factors. Bleomycin has very high intrinsic toxicity once direct access to the cell cytosol is facilitated by electric pulses [Citation5], it is present in high concentrations in the skin due to the lack of the bleomycin inactivating enzyme bleomycin hydrolase (in the skin) [Citation16]. Furthermore, electrochemotherapy has an antivascular effect causing entrapment of bleomycin in the tumour [Citation17,Citation18]. This antivascular effect combined with the anti-tumour efficacy leads to an immediate and durable effect on oozing and bleeding from the tumour, providing quick symptomatic relief [Citation19].
Evaluation of response. Previous studies on small cutaneous tumours have shown high response rates (70–90%) [Citation7,Citation8,Citation10,Citation20]. The smaller the tumours, the easier it is to cover all parts of the tumour with electric pulses. Secondly, healing requires recolonisation of normal skin from neighbouring areas, and the time this takes depends on size of the treated tumour. Finally, previous irradiation will delay healing. This means, that patients with large tumours will need a longer follow-up period in order to observe their responses, which could reflect the lower observed response rates in this and previous studies for larger tumours [Citation7,Citation21].
Response as evaluated by clinical examination was the primary endpoint in this study. This has been the method previously used for evaluation of electrochemotherapy in studies where primarily small cutaneous tumours were treated. However, this method of evaluation may lead to an underestimation of the treatment effect in larger tumours, as only a uni- dimensional measurement is used compared to volume measurement. Radiographic measurements may be more accurate and perhaps more feasible than those obtained clinically. Further studies will clarify whether PET may add useful information on metabolically active parts of the tumour that can be used when considering re-treatment of already treated lesions.
Safety. Electrochemotherapy was well tolerated although pain CTC toxicity grade III was observed in seven patients (44%). Persistent pain has not previously been reported after treatment of small tumours [Citation7,Citation8] and in our clinical experience it is only seen after treatment of large cutaneous recurrences on the chest wall. Several factors could contribute to this: 1) previous surgery and radiotherapy; 2) ulcerated lesions with chronic inflammation; 3) tumour necrosis after electrochemotherapy; and/or 4) proximity to the periost. This suggests that the treating physician should be proactive in pain treatment in this subset of patients.
A concern using bleomycin under anaesthesia has been increased lung toxicity, as bleomycin lung toxicity may be potentiated by high oxygen exposure during anaesthesia [Citation22]. In this study oxygen concentration in the inspired air was kept less than 30% during anaesthesia and DLCO was measured before and after treatment. No lung toxicity or decrease in DLCO was observed. Thus, we do not find reason for concern using bleomycin IV during general anaesthesia for electrochemotherapy when using the treatment regime described in this study.
Symptomatic relief. Electrochemotherapy is a palliative treatment, and the patients’ expressed opinion of the ability of the treatment to relieve symptoms must be taken into account. We used the DAS24 to address the effect of electrochemotherapy on self-consciousness in relation to physical appearance, but got too few data to draw conclusions. However, the patients did report reduced exudates, decreased smelling, and less bleeding along with better control of relentlessly progressing visible disease as some of the most important advantages of electrochemotherapy.
Integration with other treatments. This study is the first of its kind, and patients were generally referred when the cutaneous recurrences were very large and all other treatment options were exhausted. Single drug bleomycin has a well known and favourable toxicity profile, enabling combination with other treatment modalities. In the future, electrochemotherapy could be integrated earlier for cutaneous recurrence of breast cancer and could be given in combination with or during a pause in systemic treatment. Currently, electrochemotherapy is also under investigation for treatment of internal tumours [Citation23,Citation24].
Logistical issues. One of the concerns in palliative treatment is patients receiving time-consuming treatments and spending long hours at the hospital. Electrochemotherapy is performed within one or two hours and only one or a few sessions are needed. Compared to other treatment options such as systemic chemotherapy or multifractional irradiation, it is a relatively modest intervention. The equipment and electrodes are commercially available, as is bleomycin, and physicians can quickly learn to use the method.
Conclusion
In conclusion we find electrochemotherapy a promising treatment alternative for heavily pre-treated patients with cutaneous recurrences of breast cancer. Treatment was well tolerated and could be managed in one or few sessions. Post-treatment pain was the main side effect and symptomatic relief was obtained, in particular less odour, bleeding and/or exudate. This was a first phase II study on electrochemotherapy for chest wall recurrence of breast cancer, and further investigation is needed to explore palliative aspects of the treatment.
Acknowledgements
We would like to thank all the patients that participated in this trial. We would like to thank the staff at the Breast Cancer team at Department of Oncology together with the staff of the PET division at Department of Clinical Physiology and Nuclear Medicine, Copenhagen University Hospital Herlev for assistance. The study was investigator funded and initiated. Electrodes for the study as well as database assistance were provided by IGEA (Carpi, Italy). This work was supported by The Danish Agency for Science Technology and Innovation number 271 - 07–0513, The Research Council of Herlev Hospital, The Danish Cancer Research Foundation, Breast Friends, Agnethe Løvgreens Grant. Julie Gehl is a research fellow of the Royal Swedish Academy, supported by the Acta Oncologica Foundation.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans E, . Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;366:2087–106.
- Solin LJ, Harris EE, Orel SG, Glick J. Local-regional recurrence after breast conserving treatment or mastectomy. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the breast. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004.
- Clemons M, Hamilton T, Goss P. Does treatment at the time of locoregional failure of breast cancer alter prognosis? Cancer Treat Rev 2001;27:83–97.
- Gehl J, Skovsgaard T, Mir LM. Enhancement of cytotoxicity by electropermeabilization: An improved method for screening drugs. Anti-Cancer Drugs 1998;9:319–25.
- Orlowski S, Belehradek Jr J, Paoletti C, Mir LM. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol 1988;37:4727–33.
- Gothelf A, Mir LM, Gehl J. Electrochemotherapy: Results of cancer treatment using enhanced delivery of bleomycin by electroporation. Cancer Treat Rev 2003;29:371–87.
- Campana LG, Mocellin S, Basso M, Puccetti O, De Salvo GL, Chiarion-Sileni V, . Bleomycin-based electrochemotherapy: Clinical outcome from a single institution’s experience with 52 patients. Ann Surg Oncol 2009;16:191–9.
- Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, . Electrochemotherapy, an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. EJC Supplements 2006;4:3–13.
- Curatolo P, Quaglino P, Marenco F, Mancini M, Nardo T, Mortera C, . Electrochemotherapy in the treatment of kaposi sarcoma cutaneous lesions: A two-center prospective phase II trial. Ann Surg Oncol Epub 2011 Aug 6.
- Matthiessen LW, Chalmers RL, Sainsbury DC, Veeramani S, Kessell G, Humphreys AC, . Management of cutaneous metastases using electrochemotherapy. Acta Oncol 2011; 50:621–9.
- Whelan MC, Larkin JO, Collins CG, Cashman J, Breathnach O, Soden DM, . Effective treatment of an extensive recurrent breast cancer which was refractory to multimodal therapy by multiple applications of electrochemotherapy. EJC Supplements 2006;4:32–4.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16.
- Carr T, Moss T, Harris D. The DAS24: A short form of the Derriford Appearance Scale DAS59 to measure individual responses to living with problems of appearance. Br J Health Psychol 2005;10:285–98.
- Mir LM, Gehl J, Sersa G, Collins CG, Garbay JR, Billard V, . Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. EJC Supplements 2006;4:14–25.
- Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10.
- Ohnuma T, Holland JF, Masuda H, Waligunda JA, Goldberg GA. Microbiological assay of bleomycin: Inactivation, tissue distribution, and clearance. Cancer 1974;33:1230–8.
- Sersa G, Jarm T, Kotnik T, Coer A, Podkrajsek M, Sentjurc M, . Vascular disrupting action of electroporation and electrochemotherapy with bleomycin in murine sarcoma. Br J Cancer 2008;98:388–98.
- Gehl J, Skovsgaard T, Mir LM. Vascular reactions to in vivo electroporation: Characterization and consequences for drug and gene delivery. Biochim Biophys Acta 2002;1569:51–8.
- Gehl J, Geertsen PF. Efficient palliation of haemorrhaging malignant melanoma skin metastases by electrochemotherapy. Melanoma Res 2000;10:585–9.
- Quaglino P, Mortera C, Osella-Abate S, Barberis M, Illengo M, Rissone M, . Electrochemotherapy with intravenous bleomycin in the local treatment of skin melanoma metastases. Ann Surg Oncol 2008;15:2215–22.
- Domenge C, Orlowski S, Luboinski B, De BT, Schwaab G, Belehradek J, Jr., . Antitumor electrochemotherapy: New advances in the clinical protocol. Cancer 1996;77:956–63.
- Mathes DD. Bleomycin and hyperoxia exposure in the operating room. Anesth Analg 1995;81:624–9.
- Agerholm-Larsen B, Iversen HK, Ibsen P, Moller JM, Mahmood F, Jensen KS, . Preclinical validation of electrochemotherapy as an effective treatment for brain tumors. Cancer Res 2011;71:3753–62.
- Edhemovic I, Gadzijev EM, Brecelj E, Miklavcic D, Kos B, Zupanic A, . Electrochemotherapy: A new technological approach in treatment of metastases in the liver. Technol Cancer Res Treat 2011;10:475–85.
