Abstract
Background. We report long-term outcomes in adult patients with craniopharyngioma following surgery and radiation therapy (RT). Material and methods. Fifty-three patients treated with RT (median, 50 Gy in 25 fractions) between 1980 and 2009 with pathologically confirmed craniopharyngioma were reviewed (53% solid and 47% cystic/solid). The median age was 53 years (range, 22–76), 53% were female, 83% were sub-totally resected, 6% were gross totally resected and 11% had a biopsy and/or cyst aspiration alone. RT was delivered adjuvantly in 53% of patients as opposed to salvage intent upon progression. Results. Median follow-up was seven years (86 months, range, 8–259). The 5- and 10-year progression-free survival (PFS) rates were 85% and 69%, overall survival (OS) rates were 76% and 70%, and cause-specific survival (CSS) rates were both 88%, respectively. Both univariable and multivariable analysis identified age (<53 or ≥53) as a prognostic factor for OS (p =0.0003) and CSS (p =0.05). PFS was observed to be worse in patients with >2 surgeries prior to RT (p =0.01). Neither the intent of radiation or tumor type (cystic vs. solid/cystic) were prognostic or predictive. New endocrinopathies and visual dysfunction were observed in 53% and 17% of patients post-surgery, and in 11% and 6% post-RT, respectively. Conclusion. We report long-term favorable PFS, CSS and OS for craniopharyngioma post-RT. We observe age as a significant prognostic factor, however, timing of radiation was not.
Craniopharyngiomas pose significant management challenges to both the neurosurgeon and radiation oncologist due to the inherent surrounding critical visual, endocrinologic and vascular structures (). Although achieving a gross total resection (GTR) may yield optimal local control, a GTR is often associated with significant surgical morbidities which can seriously impair a patient’s quality of life [Citation1]. Residual disease is why radiation has been an important therapy in the management of craniopharyngioma. However, the optimal timing of radiation as either adjuvant or upon progression as a salvage therapy is unknown. Deferring radiotherapy has the potential advantages of delaying the associated long-term risks of endocrine and vascular toxicities, neurocognitive dysfunction and risk of secondary malignancy. Therefore, treatment has to be individualized based on tumor factors (location, presence of obstruction or mass effect resulting in acute neurologic compromise), patient factors (age, co-morbidities) and treatment factors (surgical accessibility), with optimal therapy being a balance between achieving the goals of local control while minimizing long-term treatment-induced sequelae. The purpose of this study was to report mature long-term outcomes specific to craniopharyngiomas post-RT, and identify prognostic factors for overall, cause-specific and progression-free survival.
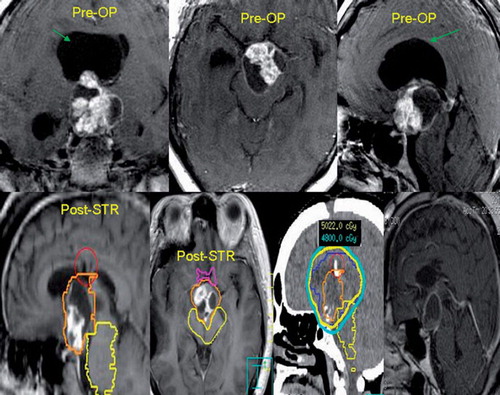
Figure 1. The top panel of images illustrates the pre-operative tumor morphology consisting of a mixed solid/cystic craniopharyngioma. The green arrow points to the large cyst responsible for the obstructive hydrocephalus resulting in this patient’s acute presentation of decreased level of consciousness. The patient underwent an emergency subtotal resection with cyst resection for decompression and the patient’s neurologic status returned to baseline. The post-operative magnetic resonance images (MRI) are shown on the bottom panel and labeled post-STR. The patient was then radiated adjuvantly with 54 Gy in 30 fractions. Typically the gross residual tumor is treated with a margin to encompass the post-operative bed. Representative isodose lines are provided on the planning CT-scan (3rd image from the left of the bottom panel). The follow-up MRI four years later indicates tumor stability (right-most image bottom panel).
Material and methods
Fifty-three adult patients with a pathologically confirmed diagnosis of craniopharyngioma were treated at our institution with RT between 1981 and 2009, and retrospectively reviewed. Baseline patient and tumor characteristics are presented in . The median total dose and number of fractions were 50 Gy and 25 fractions (range, 45–54 Gy, 20–28 fractions). Sixty-four percent of patients were treated with a conventional two-field technique, 4% with a conventional three-field technique, 26% using stereotactic arcs, and 6% with a stereotactic intensity modulated radiotherapy (IMRT) technique (the order of RT technique reflects the development of our radiation technology over the 30 year time span of this study with the latter as the most recent). No patient received chemotherapy or radionuclides either as a systemic treatment or as a locally implanted treatment.
Table I. Baseline patient and tumor characteristics.
We defined adjuvant RT as that given upfront after the first surgical resection with the intent of preventing recurrence, and delivered within six months of the primary operation with the primary tumor stable. Salvage RT was defined as that delivered in the context of imaging based tumor progression, and/or following a second debulking surgery if performed. According to these criteria, 53% of the patients were treated with adjuvant intent, and the median time to RT post-surgery was 58 days (range, 21–177).
Statistical analysis
Descriptive statistics were used to describe patient characteristics and outcome recordings. Categorical variables were expressed as count and proportions whereas continuous variables, such as follow-up in months, were expressed as means with standard deviations (SD) or medians (range) as appropriate. Cause specific survival (CSS), progression-free survival (PFS) and overall survival (OS) were calculated using the Kaplan-Meier method. Differences between survival curves were analyzed using the log-rank test. Cox proportional hazards model were used for assessing the effect of more than one potential predictor for OS, CSS, and PFS while examining the proportionality assumption. All p-values were two-sided. Results were considered significant if p ≤0.05. Statistical analyses were performed using version 9.2 of the SAS system and user's guide (SAS Institute, Inc., Cary, NC, USA).
Results
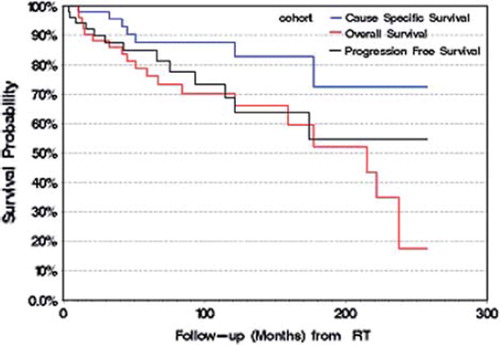
The median and mean follow-up was seven and nine years (range, 8 to 259 months) from the time of first surgery, respectively, and 5.3 and 7.5 years (range, 2 to 257 months) from the time of RT, respectively. The median survival was 19 years from time of first surgery and 18 years from the time of RT. Thirteen patients suffered from local progression following RT, the median time to recurrence was 43 months (range, 4–177), and details are summarized in . From the time of RT, the 5- and 10-year PFS rates were 85% and 69%, OS rates were 76% and 70% and CSS rates were 88% and 88%, respectively (). At last follow-up, a total of 19 patients had died. The cause of death was related to local tumor progression or treatment-related complications in four patients (4/19). The causes of death in seven patients (7/19) were identified as related to coronary artery disease (4), pulmonary embolism (1), severe Alzheimer’s disease (1), and a second unrelated brain tumor (1). In eight patients (8/19) the actual cause of death was unknown, however, records documented stability of the craniopharyngioma.
Table II. Patient and tumor characteristics of patients with local progression post-RT.
Figure 2. Overall survival, cause-specific survival and progression-free survival following radiation therapy.
Univariable and multivariable survival analysis
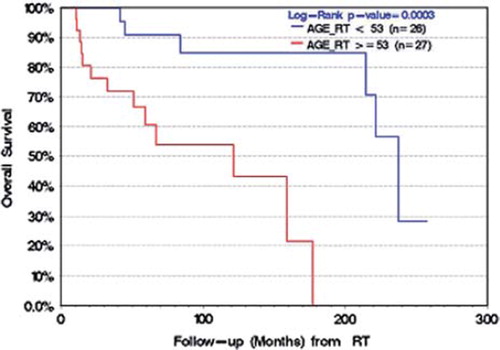
Factors included in the univariable analysis for PFS, CSS and OS were age dichotomized at the median (<53 vs. ≥53), gender, endocrine deficit at presentation, visual loss at presentation, composition of tumor, type of surgery, number of surgeries prior to radiotherapy and intent of radiotherapy (adjuvant or salvage). Univariable analysis identified age (p <0.001) as a significant prognostic factor for OS with trends for type of surgery (p =0.08), gender (p =0.1) and number of surgeries (p =0.1). Multivariable analysis identified age as the only significant prognostic factor for OS (p =0.0003, ). Univariable analysis for CSS also identified age as a significant prognostic factor (p =0.05); however, failed to meet significance upon multivariable analysis. With respect to PFS, univariable analysis determined patients having one or two surgeries prior to RT predicting for a lower risk of recurrence, as compared to patients who had undergone three resections prior to radiotherapy (p =0.01). We also observed a trend for younger age (p =0.1) as a predictive factor. However, we were unable to conclude significance of younger age on multivariable analysis. The RT technique was not a predictive factor for PFS.
Toxicity
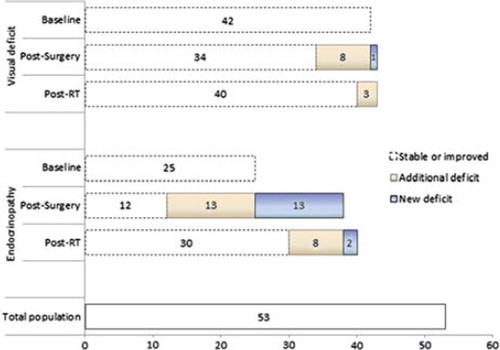
Five patients (9%) required a cyst aspiration within six months following RT. These patients were determined as pseudoprogressors given that following a simple cyst drainage procedure; all tumors remained locally controlled with no additional interventions required. We summarize the post-surgical and post-RT rates of endocrine and vision related toxicities in . Other long-term toxicities included cataracts (2%), chronic memory dysfunction (2%), cognitive defects/behavioral changes (2%), infarct within a vascular territory where major vessels had been included in the radiation volume (6%), and radiation-induced meningioma (4%).
Figure 4. Endocrine and vision related dysfunction at baseline and post-treatment according to surgery and RT. This figure presents the number of patients with either an endocrine and/or visual pathway related deficit at first presentation (Baseline). We then summarize the proportion of patients who subsequently maintained or had improvement in those deficits post-operatively (stable or improved), those whose baseline deficits deteriorated (progressive deficit) and those who developed a new deficit entirely (new deficit). We summarize the same analysis for post-RT as well.
Discussion
This series is one of the largest in the literature reporting mature outcomes post-RT for adult craniopharyngioma with a median follow-up of seven years from the time of surgical diagnosis. We observed 5- and 10-year PFS rates of 85% and 69%, OS rates of 76%, and 70% and CSS rates were both 88%, respectively. These outcomes are consistent with the limited literature [Citation2–8]. We present in the treatment course of one patient in this series to illustrate the potential for long-term favorable outcomes with a sub-total resection followed by adjuvant radiation.
Our analysis identified age ≥53 years as a significant prognostic factor for OS on multivariable analysis (p =0.0003). A similar association has been reported by Bunin et al. who identified patients aged 20–64 years having a favorable 80% 5-year OS as compared to 38% in patients ≥65 years old at diagnosis [Citation9]. Similarly, Regine et al. reported favorable 10-year OS rates for pediatric patients as compared to adult patients of 72% and 51%, respectively [Citation5]. Our CSS analysis also identified age ≥53 as prognostic on univariable analysis, but not multivariable analysis. Therefore, based on a 10-year OS of 70%, a higher 10 year CSS of 88% and age as a significant prognostic factor, we can conclude that as patients grow older they die predominantly of causes of death other than their craniopharyngioma. This finding could impact the decision of timing of RT post- operatively, in particular, as we did not observe any significant difference in OS, CSS and PFS for those patients radiated adjuvantly as opposed to with salvage intent.
A lack of association with outcomes and timing of RT has also been reported in the literature. Pemberton et al. [Citation8] compared 54 patients radiated adjuvantly to 43 radiated with salvage intent upon relapse, and concluded no significant differences in either OS or PFS at 10 years. Jose et al. [Citation10] also concluded that the timing of RT does not impact long-term outcomes. Therefore, we conclude that it is safe to observe patients following an initial sub-total resection who are neurologically stable, and reserve radiation as a salvage therapy. This may be particularly relevant for elderly patients with multiple co-morbidities and a short life expectancy.
Our analysis of PFS determined on univariable analysis that the number of surgeries pre-RT was a predictive factor. Based on a detailed review of those four patients with three surgeries prior to RT, we believe that they presented upfront with more aggressive tumor biology. A biological spectrum of craniopharyngioma tumors has been reported in the literature with even rare cases of malignant transformation [Citation11]. However, the World Health Organization (WHO) has yet to sub-categorize craniopharyngioma according to biology, and this is a subject of further study. We are in the process of studying the tissue for these cases. Of note, this factor did not maintain statistical significance in the multivariable model and, therefore, hypothesis generating until validated in larger series. We also did not observe any difference in outcomes according to tumor type, i.e. cystic vs. solid/cystic. We cannot make any conclusions for purely solid tumors as we did not have any in this series.
With respect to endocrine and visual pathway related compromise, we summarize in the number of patients with deficits observed at baseline prior to any therapy. We then summarize the number of patients following the first surgery that remained either stable or improved, the number of patients with progression of their existing deficits, and the number of patients with entirely new deficits. We describe a similar analysis according to post-RT. Our results are consistent with the literature [Citation1,Citation7,Citation12,Citation13], however, we acknowledge the inherent limitations in determining adverse events associated with a retrospective review. In particular, although most patients had serial endocrinologic and visual testing as part of their follow-up with available results for review, the frequency of follow-up tests were not uniform.
In our study, 13 patients suffered local progression (crude local failure rate of 25%). We report a comprehensive review of these cases in Table III. We observe that all patients had a STR, and approximately half were treated with salvage intent as opposed to adjuvant RT. Only three cases recurred within one year of RT, otherwise the median time to progression was approximately 6 years (1.3–14.8 years). We also observed a change in the relapse pattern for only one case (patient 13) who progressed with a cystic tumor morphology, as opposed to the initial morphology of cystic/solid tumor. Following relapse, treatment for 9/13 patients was surgery and no further RT, 1/13 had two surgical debulking procedures followed by re-irradiation and 3/13 were observed with serial imaging and no further intervention at last follow-up. Six of 13 patients are dead at last follow-up, and in five of these patients the cause of death was related to their tumor progression. The RT technique in the failure cases varied with seven patients having been treated with two-dimensional (2D) and a two-field radiotherapy technique, while the remaining six patients had 3D conformal treatment planning with multiple fields. We did not find RT technique to be a predictive factor for PFS (data not shown).
Limitations of our study include those inherent to retrospective studies and a small sample size. In particular, we could not reliably identify the craniopharyngioma subtype (adamantinomatous/papillary) for each patient, although, this factor has not been consistently associated with differences in outcomes. Additionally, our observation that the timing of RT was not prognostic or predictive may reflect a bias towards radiating those with significant residual disease or an aggressive clinical course adjuvantly, while observing those thought to harbor less aggressive disease. However, the majority of our patients were sub-totally resected, and without a randomized study we are limited to interpreting large case series to base clinical decisions upon.
Conclusion
With a median follow-up of seven years, we report favorable 5- and 10-year rates for OS, PFS and CSS. We identified age as a significant prognostic factor for OS. We did not observe any significant association for the timing of RT as either adjuvant or salvage for PFS, OS or CSS.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Sughrue ME, Yang I, Kane AJ, Fang S, Clark AJ, Aranda D, . Endocrinologic, neurologic, and visual morbidity after treatment for craniopharyngioma. J Neurooncol 2011; 101:463–76.
- Yang I, Sughrue ME, Rutkowski MJ, Kaur R, Ivan ME, Aranda D, . Craniopharyngioma: A comparison of tumor control with various treatment strategies. Neurosurg Focus 2010;28:E5.
- Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJ, . Craniopharyngioma – a long-term results following limited surgery and radiotherapy. Radiother Oncol 1993;26:1–10.
- Minniti G, Saran F, Traish D, Soomal R, Sardell S, Gonsalves A, . Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngiomas. Radiother Oncol 2007;82:90–5.
- Regine WF, Mohiuddin M, Kramer S. Long-term results of pediatric and adult craniopharyngiomas treated with combined surgery and radiation. Radiother Oncol 1993; 27:13–21.
- Schulz-Ertner D, Frank C, Herfarth KK, Rhein B, Wannenmacher M, Debus J. Fractionated stereotactic radiotherapy for craniopharyngiomas. Int J Radiat Oncol Biol Phys 2002;54:1114–20.
- Karavitaki N, Brufani C, Warner JT, Adams CB, Richards P, Ansorge O, . Craniopharyngiomas in children and adults: Systematic analysis of 121 cases with long-term follow-up. Clin Endocrinol (Oxf) 2005;62:397–409.
- Pemberton LS, Dougal M, Magee B, Gattamaneni HR. Experience of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol 2005;77:99–104.
- Bunin GR, Surawicz TS, Witman PA, Preston-Martin S, Davis F, Bruner JM. The descriptive epidemiology of craniopharyngioma. Neurosurg Focus 1997;3:e1.
- Jose CC, Rajan B, Ashley S, Marsh H, Brada M. Radiotherapy for the treatment of recurrent craniopharyngioma. Clin Oncol (R Coll Radiol) 1992;4:287–9.
- Rodriguez FJ, Scheithauer BW, Tsunoda S, Kovacs K, Vidal S, Piepgras DG. The spectrum of malignancy in craniopharyngioma. Am J Surg Pathol 2007;31:1020–8.
- Kawamata T, Amano K, Aihara Y, Kubo O, Hori T. Optimal treatment strategy for craniopharyngiomas based on long-term functional outcomes of recent and past treatment modalities. Neurosurg Rev 2010;33:71–81.
- Pereira AM, Schmid EM, Schutte PJ, Voormolen JH, Biermasz NR, van Thiel SW, . High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin Endocrinol (Oxf) 2005;62:197–204.