To the Editor,
We present a patient with coeliac disease (CD) and no other comorbidities who developed severe progressive radiation pneumonitis with fibrosis leading to respiratory failure and death after receiving curative intended concurrent chemo-radiation therapy for a local-regional advanced non-small cell lung cancer (NSCLC).
Several case reports link CD and interstitial lung diseases [Citation1–4], but a possible association between CD and the development of radiation pneumonitis has never previously been described or suggested.
Murine models show that disturbances in the lymphocyte response correlate with development of pneumonitis and lung fibrosis [Citation5–8]. CD is caused by an adverse immune response, which is also suggested as the cause of its association with different extra-intestinal autoimmune diseases [Citation9,Citation10]. Furthermore, transglutaminase 2 (TG2), which is the primary autoantigen in coeliac disease, has recently been shown to play a role in the development of pulmonary fibrosis [Citation5,Citation11].
Our hypothesis is that thoracic irradiation is more likely to trigger an adverse inflammation in patients with CD leading to pneumonitis and fibrosis.
Case presentation
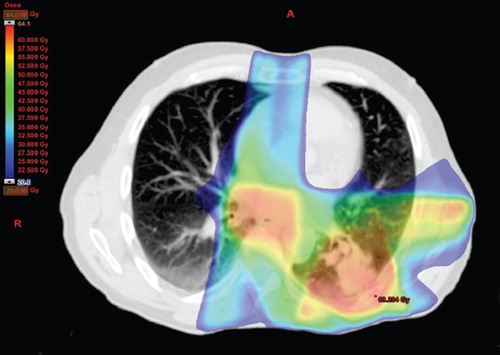
A 63-year-old male Caucasian, former smoker (25 pack-years), in good health and Performance Status (PS) = 0, was diagnosed with T2aN2M0, stage IIIA, NSCLC, adenocarcinoma in January 2011. He had no other medical history except for an episode of acute sarcoidosis with erythema nodosum, Löfgren's syndrome, in 1971 with spontaneous remission without sequelae; since 1985 biopsy- verified CD. Adhering to a gluten-free diet he had no gastro-intestinal symptoms. Before commencing treatment for lung cancer, a pulmonary function test (PFT) showed normal lung function and a slightly reduced diffusing capacity (DLCO) (); oxygen Saturation (SatO2) = 97%. The patient was included in a national phase II protocol consisting of induction chemotherapy with carboplatin and oral vinorelbine followed by concomitant oral vinorelbine and irradiation. Induction chemotherapy was started in late January 2011; the patient received full-dose, thus receiving two cycles of three weeks each; oral vinorelbine 120 mg first cycle Days 1 and 8, 160 mg second cycle Days 1 and 8 and intravenous carboplatin 650 mg on Day 1 in each cycle. In mid-March the patient commenced concomitant full-dose chemo-radiotherapy; 60 Gy in 30 fractions over six weeks with oral vinorelbine 50 mg three times a week until the end of radiotherapy in mid-April. The radiotherapy was given with a six field intensity-modulated radiation therapy (IMRT) technique (). The dosimetric factors were within the national constraints; Mean Lund Dose (MLD) = 19.6 Gy, V20 = 34.8%.
Table I. Pulmonary function tests.
One month after finishing radiotherapy, the patient presented with cough, white expectoration and a low-grade fever. Upon examination he was PS = 0, had functional dyspnea, CRP = 95 mg/L. He was treated with antibiotics for pneumonia with no effect. Two weeks later at post-therapy control, his dyspnea and cough had aggravated. Chest computed tomography (CT) revealed bilateral infiltrates (). The patient was diagnosed with radiation pneumonitis and treatment with oral prednisolone 100 mg daily was initiated. In the following two months his cough and elevated temperature subsided and CRP fell but dyspnea remained unchanged. The daily dosage of prednisolone was gradually reduced to 25 mg. CT in the period was with increased infiltration in both lung bases (). PFT showed a restrictive defect and severe diffusion impairment (). SatO2 = 89%.
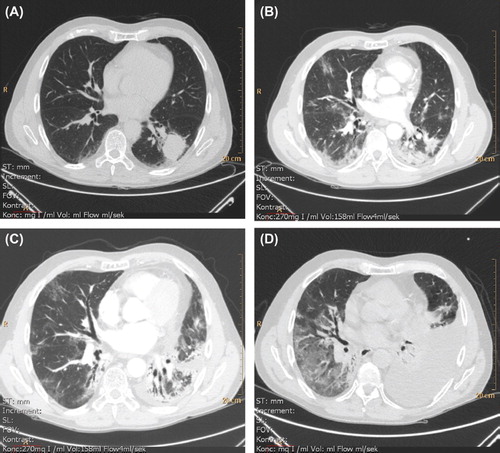
Figure 2. Radiographic (thoracic CT) development and progression of pneumonitis and pulmonary fibrosis in the period January (pre-therapy) to August (four month post-therapy) 2011. A. 4 January 2011 (without intravenous contrast). Before commencing therapy. Tumor lower left lobe. B. 30 May 2011. One month and a half post-therapy. Left lung; regression of tumor, ground-glass opacity, consolidation and air bronchogram consistent with radiation sequelae. Right lung; ground-glass opacity consistent with radiation pneumonitis. Incipient lung fibrosis with traction bronchiectasis. CTC grade 1. C. 8 July 2011. Increasing consolidation and fibrosis. CTC grade 2. D. 16 August 2011 (without intravenous contrast). Development of a left-sided pleural effusion and basal atelectasis. Right lung with increased ground-glass opacity. Fibrosis further progressed. CTC grade 3. *CTC: Common Toxicity Criteria for Adverse Events (version 4.03, 2010), pulmonary fibrosis.
In mid-August the patient was admitted acutely with exacerbated dyspnea and fever. SatO2 = 87% with 3.5 l/min nasal oxygen supplementation, temperature 39.5 C°, CRP = 110 mg/l. Treatment with intravenous broad-spectrum antibiotics and methylprednisolone 125 mg was started. During admission the patient spent three days in intensive care due to respiratory insufficiency and thoracentesis of a 1.5 l left-sided pleural exudate was performed. Analysis of the exudate revealed adenocarcinoma cells, demonstrating recurrence of the lung cancer. After 10 days he was discharged to home with continued oral prednisolone 100 mg and 3 l/min nasal oxygen supplementation. PS = 3. One week later the patient was readmitted and thoracentesis of a 1.5 l pleural exudate was again performed, with only little improvement in dyspnea. When discharged he required 5 l/min nasal oxygen supplementation at rest, becoming very dyspneic at the slightest physical activity. The patient died from respiratory failure in mid-September 2011.
Discussion
Many extra-intestinal conditions have been reported in association with CD, especially autoimmune diseases. The most frequently reported being type-I diabetes mellitus and autoimmune thyroiditis, which are more prevalent in patients with CD than in the general population. The immunological theories of these associations include sharing of common HLA genes, deviant crossing of antigens in the small intestines through the damaged mucosa, formation of autoantibodies and the deposition of immune complexes in target organs [Citation9,Citation10,Citation12].
Murine models where pulmonary inflammation is induced by bleomycin or irradiation show that lymphocytes are among the main actors of this inflammation and that disturbances in the lymphocyte response correlate with the development of pneumonitis and lung fibrosis [Citation5–8]. Keeping the high prevalence of CD (approximately 1%) in mind and that other diseases can occur concurrently simply coincidentally [Citation10], several case reports already link CD and interstitial lung diseases [Citation1–4]. Furthermore TG2, which is the primary autoantigen in CD, is recently shown to play a role in the developing of pulmonary fibrosis [Citation5,Citation11]. The case presented raises the question whether thoracic irradiation is more likely to trigger an adverse inflammation in patients with CD, leading to pneumonitis and fibrosis. It must be noted that the patient kept a gluten-free diet and had been CD-symptom- free for several years. Blood samples drawn in early September, two weeks before his death, were negative for all CD-associated antibodies including anti-TG2, indicating no current disease activity. Also angiotensin I-converting enzyme- (ACE) and calcium levels were normal. CD had been confirmed by a jejunal biopsy and the patient was identified carrying HLA-allele (DQ2) associated with CD and known to get immediate gastro-intestinal symptoms when ingesting gluten-containing foods.
The diagnosis pneumonitis with fibrosis was based on the clinical and radiographic presentation. Due to his respiratory condition and the clinical picture, a biopsy to confirm the diagnosis was not conducted. Therefore, the recurrence of lung cancer cannot be entirely ruled out as a possible differential diagnosis. At the age of 24, he experienced an episode of classical acute lung sarcoidosis, Löfgren's syndrome, which remitted spontaneously without sequelae. Sarcoidosis being a differential diagnosis for his post-therapy respiratory insufficiency is very unlikely.
So far no data suggest increased lung toxicity during concurrent vinorelbine compared to other concurrent chemo-radiotherapy regimes [Citation13].
Pneumonitis and fibrosis are known side effects to thoracic irradiation, but the rapid progress and the severity in the patient case presented is unusual. An association between CD and the development of radiation pneumonitis has to our knowledge never previously been described or suggested. Being a very common and under-diagnosed disease [Citation10,Citation12], it is of utmost importance to disclose a possible association.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Neil GA, Lukie BE, Cockcroft DW, Murphy F. Lymphocytic interstitial pneumonia and abdominal lymphoma complicating celiac sprue.J Clin Gastroenterol 1986;8:282–5.
- Hood J, Mason AM. Diffuse pulmonary disease with transfer defect occurring with coeliac disease. Lancet 1970;1: 445–7.
- Smith MJ, Benson MK, Strickland ID. Coeliac disease and diffuse interstitial lung disease. Lancet 1971;1:473–5.
- Ben-Bassat O, Lev S, Gafter-Gvili A, Katchman E, Niv Y, Fraser GM. Acute pneumonitis in a patient with celiac disease and dermatitis herpetiformis. Isr Med Assoc J 2005; 7:340–1.
- Oh K, Park H-B, Byoun O-J, Shin D-M, Jeong EM, Kim YW, . Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J Exp Med 2011;208:1707–19.
- Westermann W, Schöbl R, Rieber EP, Frank KH. Th2 cells as effectors in postirradiation pulmonary damage preceding fibrosis in the rat. Int J Radiat Biol 1999;75:629–38.
- Johnston CJ, Williams JP, Elder A, Hernady E, Finkelstein JN. Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res 2004;30:369–82.
- Cappuccini F, Eldh T, Bruder D, Gereke M, Jastrow H, Schulze-Osthoff K, . New insights into the molecular pathology of radiation-induced pneumopathy. Radiother Oncol 2011;101:86–92.
- Shaoul R, Lerner A. Associated autoantibodies in celiac disease. Autoimmun Rev 2007;6:559–65.
- Lewis NR, Holmes GKT. Risk of morbidity in contemporary celiac disease. Expert Rev Gastroenterol Hepatol 2010;4: 767–80.
- Olsen KC, Sapinoro RE, Kottmann RM, Kulkarni AA, Iismaa SE, Johnson GVW, . Transglutaminase 2 and its role in pulmonary fibrosis. Am J Respir Crit Care Med 2011; 184:699–707.
- Barker JM, Liu E. Celiac disease: Pathophysiology, clinical manifestations, and associated autoimmune conditions. Adv Pediatr 2008;55:349–65.
- Krzakowski M, Provencio M, Utracka-Hutka B, Villa E, Codes M, Kuten A, . Oral vinorelbine and cisplatin as induction chemotherapy and concomitant chemo-radiotherapy in stage III non-small cell lung cancer: final results of an international phase II trial. J Thorac Oncol 2008;3:994–1002.