To the Editor,
Bevacizumab is a recombinant humanized antibody which binds and inactivates vascular endothelial growth factor (VEGF), and thereby inhibits tumor cell proliferation. This treatment is usually associated with chemotherapy in first-line treatment of metastatic colorectal cancer [Citation1]. Side effects of bevacizumab are now well described, including gastro-intestinal (GI) perforations and cicatrization delay resulting from angiogenesis inhibition. To date, uterine perforation during treatment with bevacizumab has not been reported.
Case report
The patient, a 36-year-old female, was diagnosed a left-side colorectal cancer with hepatic and pulmonary metastasis 10 days after the end of her third pregnancy. She had no prior medical history (three pregnancies without complications). The symptoms were weight loss, asthenia and abdominal pain. The abdominal echography revealed a nodular liver, and liver biopsy diagnosed metastasis from colorectal cancer. Colonoscopy confirmed a left colonic adenocarcinoma.
Investigations for an HNPCC syndrome were negative. K-RAS gene was mutant.
An intra-uterine device (IUD) was placed soon after delivery. The patient received 22 courses of standard chemotherapy with fluorouracil, leucovorin and irinotecan (85 mg/m2) (FOLFIRI), associated with bevacizumab (5 mg/kg), from April 2010 to April 2011. Chemotherapy started one month after delivery and IUD insertion.
During the first months of the treatment, a partial response was observed. One year after diagnosis, a new evaluation CT-scan was performed and revealed a progression of the hepatic and pulmonary lesions, and a fortuitous uterine perforation (). This perforation did not exist on the 5th previous CT-scan.
Figure 1. Contrast-enhanced MDCT of the pelvis at a venous phase. Coronal reformation in the great axis of the uterus shows a perforation of the uterine fundus wall by an intrauterine contraceptive device (white arrow).
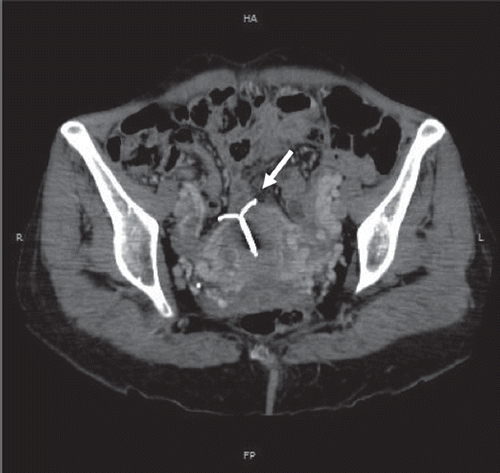
The IUD was removed three days after, without complication. The patient described no abdominal pain or gynecologic symptoms.
The endo-vaginal echography showed an uterine perforation from the endometer to the myometer, on a void uterus. The IUD was set in culture and was sterile.
Bevacizumab was first discontinued, then resumed after the first course, in association with fluorouracil, leucovorin and oxaliplatin (FOLFOX 4). The tumor progressed under this treatment and the patient died in August 2011.
Discussion
Bevacizumab is a recombinant, humanized monoclonal antibody that binds to vascular endothelial growth factor, and inhibits angiogenesis. Inhibition of VEGF signalling way in tumors results in the cessation of angiogenesis, and the regression or the normalization of some tumor vessels. It is used in combination with standard chemotherapy regimens in patients with metastatic colorectal cancer [Citation1].
The normal vasculature in adults is considered to be largely independent of VEGF for survival, but VEGF receptors are still highly expressed in several adult organs and has critical role in endothelial integrity. Endothelial fenestrations can be induced by VEGF inhibitors in vitro and in vivo [Citation2]. Bevacizumab had recently been reported to be associated with gastrointestinal perforations, fistulas and hemorrhage, most commonly within the first six month of treatment [Citation3,Citation4]. The natural history of GI perforations induced by bevacizumab is still unknown. Some mechanisms have been proposed including the disruption of the GI vasculature leading to ischemia or inhibition of healing of existing lesions.
We report the case of a 36-year-old female presenting an asymptomatic uterine perforation by an IUD as she was undergoing chemotherapy associating bevacizumab and FOLFIRI for a metastatic colorectal cancer for one year. Spontaneous uterine perforation is a rare complication of IUD (less than 0.1%) [Citation5]. They are known to remain frequently asymptomatic for a long time after IUD insertion and occur more frequently during the insertion procedure. In this observation, the role of bevacizumab in the uterine perforation cannot be proved but must be suspected, Bevacizumab could block the cicatrization of the micro-ulcerations caused by an IUD, leading to a weakened uterine wall and eventually perforation. GI perforation induced by bevacizumab is predominantly seen at the tumor or anastomostic site. Although multiple mechanisms are described in the literature the risk factors for GI perforation associated with bevacizumab are poorly understood [Citation6–9]. As no autopsy was done, we cannot know if the uterine perforation occurred at a metastatic localization.
Bevacizumab is more commonly used in various types of cancer, including breast cancer which is likely to be diagnosed in young patients. Clinicians should be aware that uterine perforation is described among patients with an IUD. More information is needed to determine whether IUD should be removed in patients undergoing a treatment by bevacizumab, in order to prevent such a complication.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, . Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–42.
- Fuchs CS, Marshall J, Barrucco J. Randomized controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Updated results from the BICC-C study. J Clin Oncol 2008;26:689–90.
- Van Cutsem E, Rivera F, Berry S, Kretzschmar A, Michael M, DiBartolomeo M, . Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyridines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009;20:1842–7.
- Hapani S, Chu D, Wu S. Risk of gastrointestinal perforation in patients with cancer treated with bevacizumab: A meta analysis. Lancet Oncol 2009;10:559–68.
- Zakin D, Stern WZ, Rosenblatt R. Complete and partial uterine perforation and embedding following insertion of intrauterine devices. Classification, complications, mechanisms, incidence and missing strings. Obstet Gynecol Surv 1981;36–7:335–53.
- Heinzerling J, Huerta, S. Bowel perforation from bevacizumab for the treatment of metastatic colon cancer: Incidence, etiology, and management. Curr Surg 2006;63:334–7.
- Schellhaas E, Loddenkemper C, Schmittel A, Buhr HJ, Pohlen U. Bowel perforation in non-small cell lung cancer after bevacizumab therapy. Invest New Drug 2008;27:184–7.
- Mir O, Mouthon L, Aleandre J, Mallion JM, Deray G, Guillevin L, . Bevacizumab-induced cardiovascular events: A consequence of cholesterol emboli syndrome? J Natl Cancer Inst 2007;99:85–6.
- Sugrue M, Kozloff M, Hainsworth J, Badarinath S, Cohn A, Flynn P, . Risk factors for gastrointestinal perforations in patients with metastatic colorectal cancer receiving bevacizumab plus chemotherapy. Proc Am Soc Clin Oncol 2006;24(18 Suppl):abstr 3535.