Abstract
Objective. To evaluate the relationship between osteopontin (OPN) in serum and plasma and parathyroid hormone-related protein (PTHrP) in serum, plasma and tumour tissue, and to assess the prognostic impact of OPN and PTHrP in human renal cell carcinoma (RCC). Material and methods. The study included 269 patients with RCC. In 189 patients, immunohistochemical (IHC) PTHrP tumour tissue expression was evaluated, and OPN and PTHrP in serum were assessed. In 80 patients, plasma OPN and PTHrP were analysed. Tumour type, TNM stage, nuclear grade and RCC-specific survival were also registered. In a sub-group, IHC expression of CD 31 was assessed. The prognostic information of the factors was analysed using uni- and multivariate analyses. Results. The median OPN level was 2.3 times higher in plasma than in serum. Serum OPN was significantly higher in patients with papillary RCC compared to clear cell RCC and chromophobe RCC. Both serum and plasma OPN levels were positively correlated to TNM stage and nuclear grade. Multivariate analysis showed that serum and plasma OPN levels were independent prognostic factors for RCC-specific survival, along with TNM stage. Immunohistochemical expression of PTHrP associated to TNM stage but not to nuclear grade or serum OPN. Furthermore, IHC expression of PTHrP was positively correlated to serum PTHrP but inversely to tumour CD31 expression. Plasma PTHrP was increased in 20% of the patients and related to TNM stage but not to nuclear grade. Plasma OPN was significantly higher in patients with increased PTHrP levels, compared to those with normal levels. Conclusion. Plasma OPN levels differed between RCC types, and in clear cell RCC, both serum and plasma OPN levels were independent predictors of survival. We found no evidence for prognostic value related to circulating levels or the IHC expression of PTHrP.
Osteopontin (OPN) is a secreted protein that belongs to the small integrine-binding ligand N-linked glycoprotein family (SIBLING) [Citation1]. The protein is expressed in a broad spectrum of normal tissue such as bone, blood vessels, epithelial cells of bronchi, gall bladder, inner ear, kidney, mammary gland, reproductive and urinary tract [Citation2].
The expression of OPN is induced by a number of stimuli such as basic fibroblast growth factor, 1,25-dihydroxyvitamin D, interleukin 1, endothelin, interferon γ, transforming growth factor β and tumour necrosis factor α [Citation3]. The signalling pathways are not fully understood.
Osteopontin is a ligand for αvβ integrins and CD44 receptor families, and activation leads to formation and remodelling of mineralised bone, regulation of immune functions, neovascularisation and impact on cell-migration and metastases [Citation4].
Necrosis is common in renal cell carcinoma (RCC), and hypoxia in the microenvironment leads to up-regulation of OPN expression via a Ras- activated enhancer [Citation5]. Osteopontin is negatively correlated to the von Hippel Lindau (vHL) gene expression [Citation6].
Several studies have demonstrated that OPN expression in tumour/plasma is related to tumour invasion, tumour progression and metastasis in different cancer forms such as breast, gastric, head-and-neck and lung carcinomas, and OPN has been identified as a strong prognostic marker in colon cancer [Citation7–11]. In RCC, OPN expression has been identified in both clear cell RCC (cRCC) and papillary RCC (pRCC) and elevated plasma levels have been associated to poor prognosis [Citation12–14].
Parathyroid hormone-related protein (PTHrP) is a poly-protein, existing in three isoforms in humans. It derives from normal and malignant cells, including RCC [Citation15–17]. The action of PTHrP is one of the main causes of hypercalcaemia in malignancies [Citation18,Citation19]. Parathyroid hormone-related protein binds to the parathyroid hormone receptor (PTH1R), and the homology to parathyroid hormone is located in the amino-terminal part of the protein [Citation20]. In addition to a role in bone re- modelling, the PTHrP/PTH1R system seems to be involved in tumour-growth regulation, and vHL tumour suppressor protein negatively regulates PTHrP expression. Furthermore, inhibition of the PTHrP/PTH1R system seems to increase microvessel density (MVD), which could suggest that PTHrP has an anti-angiogenic effect in RCC [Citation21]. The tumour expression of PTHrP was previously assessed in RCC by Gotoh et al. They showed that most tumours expressed the protein, and Iwamura et al. found that patients with tumours expressing PTHrP had longer expected relapse-free survival [Citation22,Citation23]. In RCC, we previously evaluated serum PTHrP showing that the levels were positively correlated to serum calcium levels but did not add prognostic information [Citation24].
In a rat model, where PTHrP was used to induce hypercalcaemia, Yasui et al. demonstrated that OPN expression in the kidney was enhanced in PTHrP-treated rats [Citation25]. Both OPN and PTHrP have been shown to play important roles for metastasis to bone [Citation26,Citation27]. Furthermore, there seems to be a relationship between OPN and PTH1R signalling in osteoblasts [Citation28].
The aim of the study was to evaluate the association between serum/plasma OPN and PTHrP in serum/plasma and tumour tissue, to compare the expressions of OPN and PTHrP to some previously analysed parameters with possible associations, and finally, to assess the prognostic information of OPN and PTHrP in human RCC.
Material and methods
All patients included in the study were diagnosed with RCC, at the Department of Urology, Norrland´s University Hospital, Umeå, Sweden. The patient underwent physical examination, computed tomography (CT)-scan of the chest and abdomen and if indicated skeletal scintigraphy. Histological subtypes were classified according to the Heidelberg criteria [Citation29], staging of the tumour disease was assessed according to the TNM-classification [Citation30] and nuclear grading according to Furman et al. [Citation31]. Patients with stage IV disease were mainly treated with medroxyprogesterone or interferon and eight patients received tyrosine kinase inhibitors.
Serum/plasma analyses
Serum and plasma samples were collected from patients diagnosed between 1982 and 2006. This was performed at diagnosis and before treatment, and the samples were stored at −80°C until time of analysis. Plasma samples were only collected from 2001 and onwards. In 148 patients, serum OPN was analysed and the levels were compared to the PTHrP tumour tissue expression. In 80 patients, including 44 men and 36 women with a median age of 64.5 years, diagnosed between 2001 and 2006, plasma OPN and plasma PTHrP were analysed for comparison.
The reason for the discrepancy in the number of patients in the different analyses was due to available samples at the time of analyses, as summarised in .
Table I. Summary of the materials used in the study based on a total of 269 patients with renal cell carcinoma.
Serum/plasma OPN was analysed in 2010–2011 using the Quantikine@ Human Osteopontin Immunoassay from R&D systems, Inc. (Minneapolis, MN, USA). Plasma PTHrP was analysed in 2010 using the two-site immunoradiometric assay Active@ PTHrP IRMA manufactured by Immunotech a.s (Prague, Czech Republic).
Serum PTHrP, previously analysed in 2003, in 124 of the patients in the TMA cohort, was used for comparison [Citation24]. Serum calcium (s-Ca) was analysed continuously at the time of diagnosis, according to routine procedures at the Department of Clinical Chemistry, Norrland´s University Hospital.
Tumour tissue analysis
Patients diagnosed with RCC between 1982 and 1997 were included in this cohort. In total there were 189 patients, 109 men and 80 women. The median age was 64 years. Tumour tissue samples were collected at nephrectomy and were formalin fixed and paraffin embedded.
The tumour tissues were prepared for tissue microarrays (TMA). From formalin fixed and paraffin embedded tissue blocks two tissue cores measuring 0.6 mm in diameters, representative for the tumour, were placed in a new paraffin block. Each block contained up to 49 different tumours in duplicate samples. The TMA blocks were sliced into 4 µm sections and treated according to standard procedures including de-paraffination and rehydration. A citrate buffer, pH 6.1, was used as antigen retrieval solution, and heat was applied by microwave oven for 20 minutes. Immunostaining was performed, using an automatic immunohistochemistry machine (Ventana 320 ES, Ventana Medical Systems, USA) with iView DAB detection kit. Parathyroid hormone- related protein was detected by a polyclonal anti-human guinea pig antibody T5045 (Bachem, Bubendorf, Switzerland) diluted 1:1000. Placenta and normal renal tissue were used as positive controls [Citation22,Citation32].
The immunohistochemical (IHC) expression of PTHrP was evaluated in a light microscope by one of the authors (K. P.) with assistance of another author (A. B.). The samples were coded, and information about the patients was unknown to the examiner at the time of evaluation. The immunostaining was cytoplasmatic and uniform within the tumours. The expression was categorised into three groups according to staining intensity; no (0), weak (+) and strong (++) expression. In the statistical analysis tumours with weak and strong expression were pooled and compared to tumours with no expression.
In addition, CD 31 tumour tissue expression, a marker of microvessel density, previously assessed by Sandlund et al., was used for comparison [Citation33].
Statistical analyses
Statistical analyses were performed using the computer software SPSS, version 18. Mann-Whitney U-test and Kruskal-Wallis non-parametric test were used for analysing different mean values between different groups and χ2 test was used for assessing associations between categorical variables. Correlations between different variables were assessed with Spearman correlation coefficient. Renal cell carcinoma-specific survival was analysed using the Kaplan-Meier method and survival time in different groups was compared with the log rank test. The prognostic value was assessed in a multivariate regression analysis using Cox proportional hazards model.
The study was approved by the ethical committee of Umeå University.
Results
Osteopontin was analysed in serum and plasma from 148 and 76 patients with RCC, respectively. The OPN levels in both serum and plasma were significantly higher in men compared to women (data presented as Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.693623). Furthermore, serum OPN levels were higher in patients with papillary (p)RCC compared to both clear cell (c) and chromophobe (ch)RCC (p < 0.001 and 0.009, respectively), whereas no difference was found between cRCC and chRCC. For patients with cRCC, serum and plasma OPN was positively correlated to TNM stage (p < 0.001 for both), and to nuclear grade (p = 0.036, and 0.005, respectively). No correlation between OPN and TNM stage and nuclear grade was observed in 19 patients with pRCC.
In a subset of 33 patients, OPN was analysed at one occasion to compare levels in plasma and serum. There was a fairly high correlation between plasma and serum levels of OPN (r = 0.82; p < 0.01, Spearman correlation test), and the median OPN level was 2.34 times higher in plasma than in serum.
The median follow-up time from diagnosis in patients in the serum cohort was 209 (154–330) months, and 84/148 died of RCC during follow-up. Among the patients in the plasma cohort, the median follow-up time was 75 (48–112) months, and 31/76 died of RCC. Disease-specific survival for patients with cRCC was evaluated in relation to serum and plasma OPN, divided by the median values, as shown in . Patients with OPN levels below median had a significantly longer survival time compared to those with levels above median (p < 0.001 for both cohorts), the difference remained when dividing the materials in tertiles (p = 0.001 for both cohorts). In a multivariate analysis including age, gender, TNM stage, nuclear grade and serum and plasma levels of OPN, only TNM stage and circulating OPN levels remained as independent prognostic factors in both patient cohorts ().
Figure 1. Kaplan-Meier plots of RCC-specific survival according to the median level of OPN in (A). plasma in 76 patients with cRCC diagnosed 2001–2006 (median 120 ng/mL), and (B). serum in 118 patients with cRCC diagnosed 1982–1997 (median 40 ng/mL).
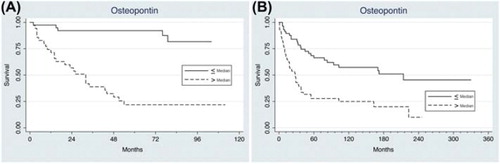
Table II. Multivariate analysis evaluating age, gender, TNM stage, nuclear grade and ostepontin (OPN) levels in relation to disease-specific survival of patients with clear cell RCC.
Plasma PTHrP levels were elevated in 16 of 80 (20%) patients with cRCC. A weak positive correlation between plasma levels of PTHrP and OPN was observed (r = 0.265; p = 0.021, Spearman correlation test). In patients with elevated circulating PTHrP, the median OPN plasma level was 243 (58–1038), compared to 103 (0.5–1275) ng/ml for those with immeasurable PTHrP in plasma (p = 0.009). However, when the same comparison was made for serum OPN, no difference between patients with measurable and non-measurable PTHrP, was found (p = 0.399). Hypercalcaemia ( > 2.60 mmol/l) was observed in 8/77 (10%) patients. In patients with hypercalcaemia, there was a significant association to plasma PTHrP (p = 0.002). There was a higher frequency of elevated plasma PTHrP in patients with TNM stages III–IV, than in stages I–II (p = 0.035; data presented as Supplementary Table II, available online at http://informahealthcare.com/doi/10.3109/0284186X.2012.693623). However, no association between PTHrP level and nuclear grade or survival time was found.
Immunohistochemical PTHrP expression was analysed in 189 patients, 153 cRCC, 23
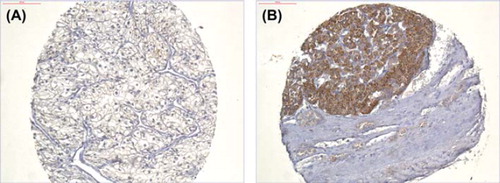
pRCC, and 13 chRCC. The staining was cytoplasmatic and uniform within the entire tissue cores (). Forty-eight percent of the tumours did not express PTHrP, 33% had weak expression and 19% had strong expression of PTHrP. No variation in expression intensity was observed between different RCC types. In patients with low TNM stages (I–II), tumour PTHrP expression was significantly more frequent than in higher stages (III–IV) (p = 0.013). There was no difference in tumour PTHrP expression between low and high nuclear grades, nor was there any difference in survival related to tumour expression of PTHrP.
Figure 2. Parathyroid hormone-related protein expression in renal cell carcinoma. No (A) and strong (B) immunohistochemical expression of PTHrP (polyclonal guinea pig antibody T5045, Bachem, Switzerland), 20 × magnification.
Serum PTHrP was elevated in 15% of the patients, and a positive correlation was observed between the serum levels and the IHC expression of PTHrP (p = 0.048, ). Furthermore, an inverse relation between tumour PTHrP expression and MVD, quantified with IHC staining for CD31, was found (p = 0.029). An inverse relation was also observed between CD 31 expression and serum OPN levels (r = −0.276, p = 0.002). Elevated serum calcium was found in 13%, but neither serum calcium nor serum OPN were related to tumour PTHrP expression.
Table III. Correlations between immunohistochemical expression of PTHrP and serum PTHrP, CD 31 tumour expression, serum calcium and serum OPN in 189 patients with renal cell carcinoma.
To evaluate stability of the proteins, the serum cohort, including patients diagnosed 1982–1997, was divided into three groups according to sample storage time (13–17, 18–22, 23–28 years, respectively). Tumour tissue IHC expression, as well as serum OPN, was stable when stored for a long time. In the plasma cohort, including patients diagnosed from 2001–2006, the material was divided in two groups according to storage time (4–6 and 7–9 years, respectively). No significant difference was observed for neither OPN nor PTHrP, thus both proteins were stable for at least 10 years in frozen plasma stored at −80°C (data not shown).
Discussion
Osteopontin and PTHrP are secreted proteins associated with cancer, and have raised interest as potential prognostic markers in different malignant disease including RCC. Both proteins are involved in bone re-modelling [Citation3,Citation15], and PTHrP has been identified as the major cause of hypercalcaemia of malignancy [Citation34]. The proteins are also involved in the regulation of tumour proliferation, angiogenesis and metastasis [Citation21,Citation35]. Results from RCC animal model studies have suggested an association between OPN and PTHrP/PTH1R-signalling [Citation25,Citation28].
Our results clearly demonstrate that circulating OPN is associated to advanced disease, high nuclear grade and shorter survival time, and is an independent prognostic factor for survival in cRCC. This confirms the results by Ramankulov et al., who found that plasma OPN was associated to distant metastasis and poor prognosis [Citation36]. In contrast to their results, we found that OPN was significantly higher in pRCC compared to other RCC types. Osteopontin is thought to partially be regulated by the VHL/HIF-system [Citation6], but our finding suggests that other mechanisms are of importance, or that hypoxia in tumour or adjacent tissue stimulate to production of OPN in pRCC. The induction of OPN by hypoxia has earlier been described [Citation37]. Furthermore, we also showed both in serum and plasma samples, that OPN levels were higher in men than in women. We have not found any literature data supporting a gender difference in OPN levels. The most probable explanation to the observed difference is that men have more advanced TNM stages of RCC compared to women. This assumption is supported by the fact that in our plasma cohort, men had significantly more advanced TNM stage compared to women (p = 0.014).
The method used for measuring OPN recommends plasma for the analysis. In the older cohort, plasma was not available, and therefore, serum was used. The OPN levels were lower in serum, but all correlations were similar and still significant, in concordance with the results in the plasma cohort. We therefore consider OPN analysis in serum possible and acceptable.
In the present study, we found that OPN levels were higher in patients with elevated PTHrP levels in the plasma cohort, and this could support a relationship between OPN and PTHrP. In the serum cohort, this association of the two proteins was not confirmed, possibly due to the fact that serum PTHrP is unstable when stored for a long time [Citation24]. Despite this, we found a positive association between serum PTHrP and the corresponding tumour tissue expression of PTHrP, which indicates that some of the circulating PTHrP derives from the renal tumour cells. Elevated levels of PTHrP in serum and plasma were seen in 15% and 20%, respectively, whereas approximately half of the tumours expressed PTHrP. This discrepancy suggests that most of the protein in tumours is not secreted, and is supported by the fact that no association between the tumour PTHrP expression and serum calcium or serum OPN was found. Our results are in line with previous observations by Gotoh et al., who found no association between PTHrP tumour expression and serum calcium levels [Citation22]. The association between circulating PTHrP and serum calcium in our study could indicate that PTHrP is produced by other tissues than renal tumour cells. Gotoh et al. also demonstrated that the expression was more frequent in pRCC compared to other RCC types. In our study, we could not show any difference in expression of PTHrP between different RCC types. The number of patients with pRCC was limited in both studies, and different anti-PTHrP antibodies were used for IHC staining, which can explain the discrepancy.
Massfelder et al. suggested that PTHrP have anti-angiogenic properties in RCC [Citation21]. We found an inverse association between PTHrP and CD 31 tumour expression, a marker of microvessel density (MVD). This observation could support PTHrP to have an inhibiting effect on angiogenesis. In RCC, low MVD has been associated to worse outcome [Citation33,Citation38]. Parathyroid hormone-related protein has been identified as a marker for poor prognosis in lung cancer [Citation39]. However, this poly-protein seems to have different functions in different tumours. In breast cancer, Henderson et al. found improved survival in patients with tumours expressing PTHrP, and Iwamura et al. showed that RCC patients with tumours expressing PTHrP had longer recurrence-free survival [Citation23,Citation40]. Yet again, a different antibody was used in their study, specific for the C-terminal of PTHrP, and a limited number was analysed. We found that tumours expressing PTHrP were more frequent in low TNM stages, which could support Iwamura's findings but we could not find any difference in survival in relation to tumour expression of PTHrP. In contrast, we found that elevated plasma PTHrP levels were more frequent in high TNM stages, although no impact on survival was demonstrated. The prognostic importance of PTHrP in human RCC remains unclear.
In summary, our results show that OPN is an independent prognostic factor in cRCC. Circulating OPN levels correlate positively to TNM stage, nuclear grade and negatively to survival time. Serum OPN levels are significantly higher in pRCC compared to other RCC types. Furthermore, the data indicate that there is a relationship between secreted OPN and PTHrP. In tumour tissue, PTHrP is associated to low TNM stage, whereas plasma PTHrP is associated to high TNM stage. Neither PTHrP tumour expression, nor circulating PTHrP are independent prognostic factors in RCC.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.693623
Download PDF (138.1 KB)Acknowledgements
This study was supported by grants from the Swedish Cancer Society, the Cancer Research Foundation in Northern Sweden, the Medical Faculty, Umeå University and the Department of Oncology, Norrland´s University Hospital, Umeå, Sweden. Thanks to Björn Tavelin for excellent assistance with statistical analysis and to Birgitta Ekblom, Jasmine Moharer and Gudrun Lindh for skilful laboratory work.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Fedarko NS, Jain A, Karadag A, Fisher LW. Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J 2004;18:734–6.
- Brown LF, Berse B, Van de Water L, Papadopoulos- Sergiou A, Perruzzi CA, Manseau EJ, . Expression and distribution of osteopontin in human tissues: Widespread association with luminal epithelial surfaces. Mol Biol Cell 1992;3:1169–80.
- Denhardt DT, Noda M. Osteopontin expression and function: Role in bone remodeling. J Cell Biochem Suppl 1998;30–31:92–102.
- Denhardt DT, Guo X. Osteopontin: A protein with diverse functions. FASEB J 1993;7:1475–82.
- Zhu Y, Denhardt DT, Cao H, Sutphin PD, Koong AC, Giaccia AJ, . Hypoxia upregulates osteopontin expression in NIH-3T3 cells via a Ras-activated enhancer. Oncogene 2005;24:6555–63.
- Le QT, Sutphin PD, Raychaudhuri S, Yu SC, Terris DJ, Lin HS, . Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res 2003;9:59–67.
- Oates AJ, Barraclough R, Rudland PS. The role of osteopontin in tumorigenesis and metastasis. Invasion Metastasis 1997;17:1–15.
- Tuck AB, Chambers AF, Allan AL. Osteopontin overexpression in breast cancer: Knowledge gained and possible implications for clinical management. J Cell Biochem 2007; 102:859–68.
- Zhang DT, Yuan J, Yang L, Guo XN, Hao ZM, Han ZY, . [Osteopontin expression and its relation to invasion and metastases in gastric cancer]. Zhonghua Zhong Liu Za Zhi 2005;27:167–9.
- Chambers AF, Wilson SM, Kerkvliet N, O’Malley FP, Harris JF, Casson AG. Osteopontin expression in lung cancer. Lung Cancer 1996;15:311–23.
- Agrawal D, Chen T, Irby R, Quackenbush J, Chambers AF, Szabo M, . Osteopontin identified as lead marker of colon cancer progression, using pooled sample expression profiling. J Natl Cancer Inst 2002;94:513–21.
- Matusan K, Dordevic G, Stipic D, Mozetic V, Lucin K. Osteopontin expression correlates with prognostic variables and survival in clear cell renal cell carcinoma. J Surg Oncol 2006;94:325–31.
- Matusan K, Dordevic G, Mozetic V, Lucin K. Expression of osteopontin and CD44 molecule in papillary renal cell tumors. Pathol Oncol Res 2005;11:108–13.
- Ramankulov A, Lein M, Kristiansen G, Meyer HA, Loening SA, Jung K. Elevated plasma osteopontin as marker for distant metastases and poor survival in patients with renal cell carcinoma. J Cancer Res Clin Oncol 2007;133:643–52.
- Strewler GJ. The physiology of parathyroid hormone-related protein. N Engl J Med 2000;342:177–85.
- Danks JA, Ebeling PR, Hayman J, Chou ST, Moseley JM, Dunlop J, . Parathyroid hormone-related protein: Immunohistochemical localization in cancers and in normal skin. J Bone Miner Res 1989;4:273–8.
- Tsuchihashi T, Yamaguchi K, Miyake Y, Otsubo K, Nagasaki K, Honda S, . Parathyroid hormone-related protein in tumor tissues obtained from patients with humoral hypercalcemia of malignancy. J Natl Cancer Inst 1990;82:40–4.
- Honda S, Yamaguchi K, Suzuki M, Sato Y, Adachi I, Kimura S, . Expression of parathyroid hormone-related protein mRNA in tumors obtained from patients with humoral hypercalcemia of malignancy. Jpn J Cancer Res 1988;79:677–81.
- Burtis WJ, Brady TG, Orloff JJ, Ersbak JB, Warrell RP, Jr., Olson BR, . Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med 1990;322: 1106–12.
- Grill V, Rankin W, Martin TJ. Parathyroid hormone-related protein (PTHrP) and hypercalcaemia. Eur J Cancer 1998; 34:222–9.
- Massfelder T, Lang H, Schordan E, Lindner V, Rothhut S, Welsch S, . Parathyroid hormone-related protein is an essential growth factor for human clear cell renal carcinoma and a target for the von Hippel-Lindau tumor suppressor gene. Cancer Res 2004;64:180–8.
- Gotoh A, Kitazawa S, Mizuno Y, Takenaka A, Arakawa S, Matsumoto O, . Common expression of parathyroid hormone-related protein and no correlation of calcium level in renal cell carcinomas. Cancer 1993;71:2803–6.
- Iwamura M, Wu W, Muramoto M, Ohori M, Egawa S, Uchida T, . Parathyroid hormone-related protein is an independent prognostic factor for renal cell carcinoma. Cancer 1999;86:1028–34.
- Papworth K, Grankvist K, Ljungberg B, Rasmuson T. Parathyroid hormone-related protein and serum calcium in patients with renal cell carcinoma. Tumour Biol 2005; 26:201–6.
- Yasui T, Fujita K, Sasaki S, Iguchi M, Hirota S, Nomura S, . Alendronate inhibits osteopontin expression enhanced by parathyroid hormone-related peptide (PTHrP) in the rat kidney. Urol Res 1998;26:355–60.
- Allan A, Tuck A, Bramwell V, Vandenberg T, Winquist E, Chambers A. Contribution of osteopontin to the development of bone metastasis. Bone Metastasis 2005:107–23.
- Liao J, McCauley LK. Skeletal metastasis: Established and emerging roles of parathyroid hormone related protein (PTHrP). Cancer Metastasis Rev 2006;25:559–71.
- Ono N, Nakashima K, Rittling SR, Schipani E, Hayata T, Soma K, . Osteopontin negatively regulates parathyroid hormone receptor signaling in osteoblasts. J Biol Chem 2008;283:19400–9.
- Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, . The Heidelberg classification of renal cell tumours. J Pathol 1997;183:131–3.
- Sobin L WC, International Union Against Cancer (UICC), editor. TNM classification of malignant tumours, 6th ed. Hoboken, NJ: John Wiley & Sons; 2002.
- Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol 1982;6:655–63.
- Emly JF, Gregory J, Bowden SJ, Ahmed A, Whittle MJ, Rushton DI, . Immunohistochemical localization of parathyroid hormone-related protein (PTHrP) in human term placenta and membranes. Placenta 1994;15:653–60.
- Sandlund J, Hedberg Y, Bergh A, Grankvist K, Ljungberg B, Rasmuson T. Evaluation of CD31 (PECAM-1) expression using tissue microarray in patients with renal cell carcinoma. Tumour Biol 2007;28:158–64.
- Budayr AA, Nissenson RA, Klein RF, Pun KK, Clark OH, Diep D, . Increased serum levels of a parathyroid hormone-like protein in malignancy-associated hypercalcemia. Ann Intern Med 1989;111:807–12.
- Wai PY, Kuo PC. Osteopontin: Regulation in tumor metastasis. Cancer Metastasis Rev 2008;27:103–18.
- Ramankulov A, Lein M, Kristiansen G, Loening SA, Jung K. Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate 2007;67:330–40.
- Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev 2004;23:293–310.
- Delahunt B, Bethwaite PB, Thornton A. Prognostic significance of microscopic ascularity for clear cell renal cell carcinoma. Br J Urol 1997;80:401–4.
- Hiraki A, Ueoka H, Bessho A, Segawa Y, Takigawa N, Kiura K, . Parathyroid hormone-related protein measured at the time of first visit is an indicator of bone metastases and survival in lung carcinoma patients with hypercalcemia. Cancer 2002;95:1706–13.
- Henderson M, Danks J, Moseley J, Slavin J, Harris T, McKinlay M, . Parathyroid hormone-related protein production by breast cancers, improved survival, and reduced bone metastases. J Natl Cancer Inst 2001;93:234–7.