Abstract
Background. This study aims at comparing the morbidity and oncologic outcomes in normal weight, overweight, and obese women with locally advanced cervical cancers (LACC) submitted to radical surgery after chemoradiation. Methods. A review of LACC patients with body mass index (BMI) ≥18.5 kg/m2 who underwent neoadjuvant chemoradiation followed by radical surgery between January 1996 and December 2010 was performed. BMI categories were created according to the World Health Organization (WHO) classification. Results. Two hundred sixty-eight women met the inclusion criteria: 118 (44.0%) were normal weight, 100 (37.3%) overweight and 50 (18.7%) obese. The median follow-up was 42 months. Higher BMI was associated with older age (p = 0.0041), while there were no differences among the three groups in Charlson comorbidity score, tumor characteristics, radiotherapy dosing, type of surgery, and pathological response. There were no differences among the three groups in the intraoperative and postoperative complications as well as rate of patients requiring adjuvant treatments: 21 (7.8%) patients experienced grade 3–4 toxicity, including six normal weight, 12 overweight and three obese patients (p = 0.14). Only the rate of grade 1–2 skin toxicity was higher in obese (14%) with respect to overweight (1%) and normal women (0%) (p = 0.00001). There were no differences in the five-year DFS (74%, 77%, and 84% for normal weight, overweight, and obese women, respectively, p = n.s.), and five-year OS (76%, 78%, and 78% for normal weight, overweight, and obese women, respectively, p = n.s.). Conclusions. The role of obesity should not be overestimated when evaluating the chance of enrolment of LACC patients into preoperative chemoradiation protocols.
During the last decades a dramatic increase in obesity rates has been observed in developed countries, where it has become a growing health and economic problem. Indeed, obesity is associated with different morbid conditions (i.e. cardiovascular diseases, diabetes, metabolic and hypoventilation syndromes) and it may even account for 20% of cancer deaths among women, representing the largest preventable cause of cancer among non-smokers [Citation1,Citation2]. Indeed, breast, colon, ovarian, endometrial and pancreatic cancers, which are the leading causes of cancer mortality among women, are associated with an increased incidence in the obese population, as a result of possible hormonal effects (e.g. increased levels of unopposed estrogens, insulin, insulin-like growth factor I), and/or delay in diagnosis (i.e. uncompliance to screening procedures) [Citation1,Citation2]. In addition, an elevated body mass index (BMI) is associated with higher death rates due to cancers of the esophagus, colon and rectum, liver, gallbladder, pancreas, kidney, non-Hodgkin lymphoma, and multiple myeloma, which could reflect an increased cancer incidence in obese women and/or a worse prognosis as a consequence of more advanced stages and inappropriate treatment [Citation1]. Among gynecologic malignancies, significant trends for an increased risk of death among women with an elevated BMI have been reported for breast and endometrial cancer [Citation1], while data are less consistent for ovarian cancer [Citation1,Citation2]. Similarly, the prognostic role of obesity is still undefined in cervical cancer: while population-based studies had suggested increased mortality with higher BMI [Citation1,Citation3], more recent studies, specifically focused on early stage and locally advanced cervical cancer (LACC) patients, reported that a high BMI does not adversely influence the prognosis in women submitted to radical surgery [Citation4] and definitive chemoradiation, respectively [Citation5].
In order to maximize local control and eventually improve survival in the LACC subset of patients, the use of a three-modality treatment, including completion surgery after concurrent chemoradiation, has gained attention during the last decade: this tri-modality approach resulted in disease-free and overall survival rates at least comparable to exclusive chemoradiation, with a different long-term toxicity profile [Citation6,Citation7]. However, this approach might raise some concerns especially in “high-risk” patients; in particular, up till now, the potential impact of obesity on the toxicity and efficacy of this multimodal strategy has not been investigated in LACC. Besides concerns about the appropriate radiation dosimetry and chemotherapy dosing to control chemoradiation toxicity, obese women are often considered poor candidates for radical surgery, not only for the perception of a more difficult operative technique: many gynecologic oncologists believe that a high BMI is associated with increased rate of intraoperative and postoperative complications, and most of them perceive obesity as a prognostic negative factor which may compromise the “radicality” of surgical procedure, in terms of less extended colpectomy and parametrectomy, as well as the extent of lymphadenectomy, even though prior studies, focused on primary surgery, have not shown this to be true [Citation4,Citation8–11].
The objective of the present study is to compare the morbidity and the oncologic outcomes in terms of pathological responses, disease-free survival (DFS) and overall survival (OS), in normal weight, overweight, and obese women with LACC submitted to radical surgery after chemoradiation.
Patients and methods
This study includes 292 consecutive LACC patients, accrued between January 1996 and December 2010 at the Gynecologic Oncology Units of the Catholic University of Rome and Campobasso, who were triaged to our preoperative chemoradiation protocol [Citation7]. Inclusion criteria for our chemoradiation plus surgery protocol were the following: biopsy-proven carcinoma of the cervix Federation Internationale de Gynecologie et d’Obstetrique (FIGO) stage Ib2–IVa, without evidence of disease outside the pelvis; age ≤80 years; Eastern Cooperative Oncology Group performance status <2; adequate bone-marrow function (WBC > 3000, platelets >120,000/mm3); adequate renal function (blood urea nitrogen <25 mg/dl, creatinine <1.5 mg/dl); normal liver function (bilirubin <2 mg/dl) and no prior cancer other than basal cell carcinoma [Citation7]. All patients signed a written informed consent agreeing to be submitted to all the procedures described and for their data to be collected. This study was approved by the Institutional Ethical Committee of the Catholic University of Rome. Pretreatment work up included a medical history, clinical examination, chest radiography, abdominopelvic magnetic resonance imaging (MRI), complete blood count and measurement of liver and renal function; cystoscopy and proctoscopy were performed if there was a clinical suspicion of invasion. We reviewed the records of all patients with BMI ≥ 18.5 kg/m2 who underwent neoadjuvant chemoradiation followed by radical surgery for LACC.
The BMI for each patient was determined by dividing her weight in kilograms by the square of her height in meters. Weight and height were measured for each patient before the first chemotherapy cycle. The World Health Organization defines a normal body weight as a BMI from 18.5 kg/m2 to 24.9 kg/m2, underweight as a BMI <18.5 kg/m2, overweight as a BMI from 25.0 kg/m2 to 29.9 kg/m2, and obesity as a BMI ≥ 30 kg/m2. We divided our study population into three categories of normal weight, overweight and obese. Medical comorbidities were assigned a score in the manner described by Charlson et al. [Citation12].
Neoadjuvant chemoradiotherapy with external radiotherapy plus concomitant cisplatin-5-fluorouracil-based chemotherapy was administered according as follows: most of the patients (n = 118) were submitted to the whole pelvic irradiation in 22 fractions (1.8 Gy/day, totaling 39.6 Gy) in combination with cisplatin (20 mg/m2, 2-h intravenous infusion) and 5-fluorouracil (1000 mg/m2, 24-h continuous intravenous infusion) (both on days 1–4 and 27–30) as previously described. Slightly different schemes of pelvic radiation administration were employed in patients enrolled in satellite studies aimed at increasing the total dose of irradiation: in particular, in 66 patients, besides the above reported regimen, a dose escalation of external radiotherapy was delivered on the primary tumor through the concomitant boost technique (0.9 Gy per fraction), thus delivering three different dose levels with total doses ranging from 43.2 Gy through 46.8 Gy to 50.4 Gy. Twenty-five patients were submitted to the whole pelvic irradiation in 25 fractions (2.0 Gy/day, on weeks 1–2, 5–6, 9, totaling 50 Gy) [Citation15]. Finally, 59 patients underwent pelvic irradiation in 20 fractions (total dose from 40/2 Gy to 50/2.5 Gy) during four weeks [Citation16].
Four weeks after the end of concomitant chemoradiotherapy, patients were evaluated for objective response which was recorded according to the Response Evaluation Criteria in Solid Tumors (RECIST) [Citation17]. With the exception of patients progressing during treatment, all cases were triaged to radical hysterectomy according to Piver et al. [Citation18] and pelvic ± aortic lymphadenectomy, five to six weeks after the end of chemoradiation.
The type of radical hysterectomy performed was at surgeon's discretion, depending on the extension of disease at the time of surgery. Laparotomy with a vertical skin incision was used in the vast majority of the patients with the exception of eight patients, operated in the last year, in which a laparoscopic approach was used. Women with positive pelvic nodes at frozen section or suspicious nodes at imaging/palpation were submitted to aortic lymphadenectomy upper to the level of the inferior mesenteric artery.
Surgical morbidity was classified according to Chassagne grading system [Citation19]. Early postoperative and long-term complications were defined as any adverse event occurring within or after 30 days from surgery, respectively. After surgery, patients underwent physical examination, complete blood count and blood chemistry every three months for the first two years and every six months thereafter. Chest radiography and abdominopelvic MRI or computed tomography (CT)-scan were performed every six months for the first three years and every 12 months thereafter.
Pathologic response was defined as previously reported [Citation7]. In particular, complete response included cases with absence of any residual tumor after treatment at any site level, and microscopic partial response included cases with persistence of only microscopic foci (≤3 mm maximum dimension) at any site levels.
The χ2 and the Kruskall Wallis tests were used to analyze the possible correlation between different clinico-pathological variables and BMI groups. DFS was calculated from the date of surgery to the date of relapse or the date of the last follow-up, and overall survival OS was calculated from the date of diagnosis to the date of death or the date of the last follow-up. Medians and life tables were computed using the product limit estimate by Kaplan-Meier methods [Citation20] and the log-rank test was used to assess the statistical significance [Citation21]. Statistical analysis was carried out by using SOLO (BMDP Statistical Software, Los Angeles, CA, USA).
Results
Within the study period 292 patients initially were identified. Among these, 11 patients were excluded because a BMI < 18.5 kg/m2, while 13 patients were excluded because unable to undergo surgery for the following reasons: disease progression during radiochemotherapy (n = 5, all normal weight), severe toxicity during radiochemotherapy (n = 4, 1 normal, 3 overweight) or surgery refusal (n = 4, 3 normal, 1 obese). In particular, when analyzed by weight group, 7.6% of normal weight women (n = 9), 3.0% of overweight women (n = 3) and 2.0% of obese women (n = 1) were not operated (p = n.s.). In the remaining 268 patients, a median BMI of 25.7 kg/m2 (range 18.6–41.2 kg/m2) was found. Of these 118 (44.0%) were normal weight (median BMI = 22.1, range 18.6–24.7 kg/m2), 100 (37.3%) were overweight (median BMI = 27.2, range 25.0–29.9 kg/m2) and 50 (18.7%) were obese (median BMI = 32.6, range 30.0–41.2 kg/m2).
The distribution of patient and clinical characteristics across different BMI groups are shown in . Because of missing data points, the totals for some categories of data presented are less than 268. Higher BMI was associated with older age with a median age of 49 in normal weight women, 53 in overweight women and 57 in obese women (p = 0.0041). There were no significant differences among the three groups in Charlson score as well as with regard to tumor characteristics such as histotype, grade, FIGO stage, tumor size, or suspicious lymph node involvement at imaging. Similarly, there were no significant differences among the three groups with regard to the dose of radiotherapy administered and the clinical response. Most of the patients underwent to type III–IV hysterectomy, which was performed in 78%, 68%, and 72% of normal, overweight, and obese women, respectively (p = 0.89). While all the patients underwent pelvic lymphadenectomy, aortic lymphadenectomy was performed in 35.6%, 40%, and 24% of normal, overweight, and obese women, respectively (p = 0.16). As reported in , on pathologic examination, no significant differences were observed among the three groups in the median number of pelvic lymph node removed, whereas a slightly higher number of aortic lymph nodes were removed in the normal eight (median number = 13.5) versus overweight (median number = 9.0) and obese women (median number = 9.5) (p = 0.075). There were no differences among the three groups with regard to the pathological response to chemoradiation and the pathological involvement of the lymph nodes. Intraoperatively, there were no significant differences among the three groups in the rate of overall complications (all managed during surgery with no major intervention), estimated blood loss, blood transfusions, or operative time (). Postoperatively, there were no significant differences among the three groups with regard to the days of hospital stay, the febrile morbidity (>38°C for 2 days), the rate of early and late complications and the rate of patients requiring adjuvant treatments (). As shown in , no significant differences were observed among the three group in the rate of urinary, gastrointestinal, vascular, genital, neurological, pelvic or other systemic (i.e. sepsis or cardiac failure) morbidities; on the contrary, skin toxicity (i.e. wound dehiscence) was more frequent in obese (14%) with respect to overweight (1%) and normal weight women (0%) (p = 0.00001). Overall, 21 (7.8%) patients experienced grade 3–4 toxicity, including six normal, 12 overweight and three obese patients (5.1% vs. 12% vs. 6%, respectively; p = 0.14). Among these, five (1.9%) treatment-related deaths were recorded: one overweight woman died of acute renal failure (after two weeks from surgery), one normal and one overweight women died of late bowel perforation (after three and 48 months from surgery, respectively), one overweight woman died of late pulmonary embolism, and one obese woman with nephrostomy died of late urosepsis (after 10 years from surgery).
Table I. Preoperative characteristics of the whole study population (n = 268).
Table II. Surgical and pathological details in the whole study population (n = 268).
Table III. Postoperative characteristics of the whole study population (n = 268).
Table IV. Complications detailed according to Chassagne classification in the whole population (n = 268).
The median duration of follow-up in the study population was 42 months (range 4–181). Fifty-four (20.1%) recurrences, 52 (19.4%) deaths from any cause, and 44 (16.4%) deaths for disease were observed in the study population. The five-year DFS, five-year OS, and five-year disease-specific survival for the whole population were 76.8%, 77.0% and 79.2%.
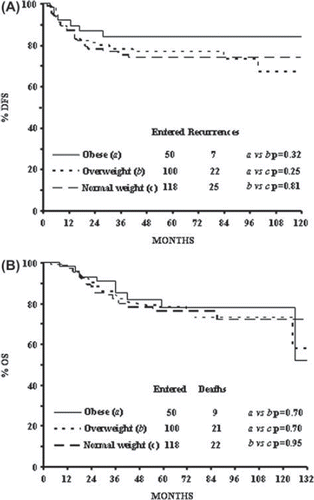
There was no significant difference among the three groups in recurrence rate: 21.2% for normal weight women, 22.0% for overweight women, and 14.0% for obese women (p = n.s.). The five-year DFS for normal, overweight, and obese women were 74%, 77%, and 84%, respectively, while the median DFS in each group was not reached ().
Death from any cause was observed in 22 (18.6%) normal weight women, 21 (21.0%) overweight women, and 9 (18.0%) obese women (p = n.s.). The five-year OS for normal, overweight, and obese women were also similar, being 76%, 78%, and 78%, respectively, while the median OS in each group was not reached (). Similarly there were no significant differences in the rate of deaths for disease and in disease-specific survival between the three groups (data not shown).
Similar results about the distribution of clinical characteristics, treatments, pathological responses, toxicities and DFS and OS measures according to BMI groups were observed by considering the 14 morbidly obese women (BMI ≥ 35kg/m2, 28% of our obese population), as a fourth BMI category (data not shown).
Discussion
This is the first study aimed at evaluating the impact of obesity in a large series of LACC patients submitted to completion surgery after chemoradiation and characterized by a long-term follow-up of oncologic outcome measures and treatment-related sequelae.
To date more than 20 studies have investigated preoperative chemoradiation in more than one thousand patients: all authors have administered radiation at a cumulative dose of 40–80 Gy, with cisplatin-based concomitant chemotherapy, reporting outcome measures at least comparable to the efficacy results of exclusive chemoradiation (57–85% for median DFS and 64–90% for median OS) [Citation7]. The clinical benefit of completion surgery, however, has been debated together with the potential risk of more frequent and/or severe toxicity, which is considered an intrinsic feature of every multimodal approach [Citation22]. In particular, it is undeniable that this approach might raise some concerns especially in obese women, and consequently lead to patient exclusion and undertreatment, as well as to limitation of generalizability of this strategy to all patients. Our study, which reports data relative to the largest single-institutional series of LACC patients treated with this three-modality approach, suggests that obesity should not be considered an absolute contraindication to this strategy.
Indeed, the toxicity rates and the severity of complications were similar among obese, overweight and normal weight patients, thus confirming in this selected patient population, prior literature data reported for primary radical surgery [Citation4,Citation8–11]. Undoubtedly, the technical and cultural advancements obtained in the last decades in surgery as well as in chemoradiation administration, together with the ameliorated prevention and management of complications might have contributed to the excellent results obtained in the obese population. In particular, only 6% of obese women experienced grade 3–4 treatment-related sequelae in our LACC series: the only one complication associated with obesity was cutaneous toxicity, which has been already related to high BMI [Citation23,Citation24], with grade 1–2 laparotomic suture dehiscence retrieved in 14% of obese women. In the last years, the introduction of minimally invasive techniques, such as conventional or robot-assisted laparoscopy, in the surgical management of cervical cancer is expected to drastically reduce this adverse event also in patients administered chemoradiation [Citation25].
The absence of a detrimental effect of obesity is particularly important, considering the more advanced age of obese women with respect to the overweight and normal weight ones, a finding which has been already reported in cervical cancer [Citation26].
More important, obesity did not show any adverse prognostic influence in this specific population. Indeed, no differences were found among the three BMI groups in adverse tumor characteristics, such as the incidence of the adenocarcinoma histotype or the distribution of more advanced FIGO stage, although both features appear to be associated with obesity, because of hormonal effects and non-compliance with screening pap tests [Citation2]. Similarly, we did not find any differences among the three BMI groups in the radicality of surgery (i.e. type of hysterectomy, level of lymphadenectomy, number of lymph nodes resected), as well as radiotherapy dosing, pathological response to chemoradiation or use of adjuvant therapies. As a consequence, our patients received a relatively homogeneous approach, thus minimizing the potential prognostic bias related to the exclusion of obese women from adequate treatment because of poor access to health care or fear of complications, as reported in other studies [Citation1,Citation4,Citation8].
Taking into account these considerations, the presence of obesity appears not to influence clinical outcome in terms of DFS and OS. Previous literature relative to cervical cancer outcome and BMI is heterogeneous: two large population-based studies reported an increased mortality with body weight increases [Citation1,Citation3], possibly as a consequence of obese patients’ low socioeconomic status and poor access to screening and treatments [Citation27], while a large retrospective study showed that a higher BMI was associated with a more favorable prognosis [Citation26]. Such controversial results might be due to a selection bias as well as lack of information relative to factors that deeply influence clinical outcome, such as stage distribution and type of treatment. On the other hand, in the homogeneous subset of early cervical cancer patients submitted to radical hysterectomy, obesity was shown not to have any detrimental effect on survival in five retrospective studies [Citation4,Citation8–11]. Similar results were reported by Kizer et al. in the setting of LACC patients administered exclusive chemoradiation [Citation5].
Our results are unlikely to be conditioned by the preselection bias of “healthier” patients, since BMI was not among the eligibility criteria for our chemoradiation protocols, and only one out of 13 patients unable to undergo surgery was obese. Moreover, although the limitations of the small sample series and the Mediterranean nature of our population could limit the generalizability of conclusions, the lack of detrimental effects of BMI on toxicity and outcome measures was also demonstrated in morbidly obese women.
In conclusion, considering the good oncologic outcome measures and the low complication rate, the role of obesity should not be overestimated in the treatment decision-making when evaluating the chance of enrolment of LACC patients into this trimodal therapeutic strategy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ.Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults.N Engl J Med2003;348:1625–38.
- Modesitt SC, van Nagell Jr JR.The impact of obesity on the incidence and treatment of gynecologic cancers: A review.Obstet Gynecol Surv2005;60:683–92.
- Garfinkel L.Overweight and cancer.Ann Intern Med1985;103:1034–6.
- Frumowitz M, Charlotte CS, Jhingran A, Schmeler KM, dos Reis Ricardo, Milam MR, et al.Radical hysterectomy in obese and morbidly obese women with cervical cancer.Obstet Gynecol2008;112:899–905.
- Kizer NT, Thaker PH, Gao F, Zighelboim I, Powell MA, Rader JS, et al.The effects of body mass index on complications and survival outcomes in patients with cervical carcinoma undergoing curative chemoradiation therapy.Cancer2011;117:948–56.
- Ferrandina G, Legge F, Fagotti A, Fanfani F, Distefano M, Morganti A, et al.Preoperative concomitant chemoradiotherapy in locally advanced cervical cancer: Safety, outcome, and prognostic measures.Gynecol Oncol2007;107(1 Suppl 1):S127–32.
- Ferrandina G, Margariti PA, Smaniotto D, Petrillo M, Salerno MG, Fagotti A, et al.Long-term analysis of clinical outcome and complications in locally advanced cervical cancer patients administered concomitant chemoradiation followed by radical surgery.Gynecol Oncol2010;119:404–10.
- Finan MA, Hoffman MS, Chambers R, Fiorica JV, DeCesare S, Kline RC, et al.Body mass predicts the survival of patients with new International Federation of Gynecology and Obstetrics stage IB1 and IB2 cervical carcinoma treated with radical hysterectomy.Cancer1998;83:98–102.
- Cohn DE, Swisher EM, Herzog TJ, Rader JS, Mutch DG.Radical hysterectomy for cervical cancer in obese women.Obstet Gynecol2000;96:727–31.
- Levrant SG, Frutcher RG, Maman M.Radical hysterectomy for cervical cancer: Morbidity and survival in relation to weight and age.Gynecol Oncol1992;45:317–22.
- Soisson AP, Soper JT, Berchuck A, Dodge R, Clarke-Pearson D.Radical hysterectomy in obese women.Obstet Gynecol1992;80:940–3.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR.A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation.J Chron Dis1987; 40:373–83.
- Mancuso S, Smaniotto D, Benedetti Panici P, Favale B, Greggi S, Manfredi R, et al.Phase I–II trial of preoperative chemoradiation in locally advanced cervical carcinoma.Gynecol Oncol2000;78:324–8.
- Cellini N, Smaniotto D, Scambia G, Luzi S, Balducci M, Ferrandina G, et al.Chemoradiation with concomitant boost followed by radical surgery in locally advanced cervical cancer: A dose-escalation study.Am J Clin Oncol2008; 31:280–4.
- Macchia G, Ferrandina G, Legge F, Deodato F, Ruggieri V, Lorusso D, et al.Prolonged chemoradiation in locally advanced carcinoma of the uterine cervix: Final results of a phase II study (ESTER-1).Am J Clin Oncol2010;33:577–82.
- Macchia G, Ferrandina G, Deodato F, Ruggieri V, Massaccesi M, Salutari V, et al.Concomitant boost dose escalation plus large-field preoperative chemoradiation in locally advanced carcinoma of the uterine cervix: Results of a phase I study (LARA-CC-1).Gynecol Oncol2010;118:128–33.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al.New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada.J Natl Cancer Inst2000;92:205–16.
- Piver MS, Rutledge F, Smith JP.Five classes of extended hysterectomy for women with cervical cancer.Obstet Gynecol1974;44:265–72.
- Chassagne D, Sismondi P, Horiot JC, Sinistrero G, Bey P, Zola P, et al.A glossary for reporting complications of treatment in gynecological cancers.Radiother Oncol1993;26:195–202.
- Kaplan FL, Meier P.Non parametric estimation from incomplete observations.Am J Stat Assoc1958;53:457–81.
- Mantel N.Evaluation of survival data and two new rank order statistics arising in its consideration.Cancer Chemother Rep1966;50:163–70.
- Jones B.Toxicity after cervical cancer treatment using radiotherapy and chemotherapy.Clin Oncol2009;21:56–63.
- Alexander CI, Liston WA.Operating on the obese woman – a review.BJOG2006;113:1167–72.
- von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR.Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma: A Gynecologic Oncology Group study.Cancer2006;107:2786–91.
- Colombo PE, Bertrand MM, Gutowski M, Mourregot A, Fabbro M, Saint-Aubert B, et al.Total Laparoscopic radical hysterectomy for locally advanced cervical carcinoma (stages IIB, IIA and bulky stages IB) after concurrent chemoradiation therapy: Surgical morbidity and oncologic results.Gynecol Oncol2009;114:404–9.
- Münstedt K, Wagner M, Kullmer U, Hackethal A, Franke FE.Influence of body mass index on prognosis in gynecological malignancies.Cancer Causes Control2008;19: 909–16.
- Maruthur NM, Bolen SD, Brancati FL, Clark JM.The association of obesity and cervical cancer screening: A systematic review and meta-analysis.Obesity (Silver Spring)2009;17: 375–81.