Abstract
Aim. The aim was to assess feasibility of a 3 + 1 week inpatient rehabilitation program for cancer survivors, to explore characteristics of the attending participants and examine changes in work status, symptoms and functioning, level of fatigue, exercise and physical performance following rehabilitation. Methods. This was an open intervention study involving cancer survivors having completed primary cancer treatment. The multidisiplinary program consisted of physical training, patient education and group sessions. Participant were assessed at primary stay (T0), at follow-up stay 8–12 weeks later (T1), and six months after T1 (T2). Symptoms and functioning were assessed by the European Organization for Research and Treatment Core Quality-of-Life Questionnaire, physical fatigue by Fatigue Questionnaire, physical exercise by The Nord- Trøndelag Health Study Physical Activity Questionnaire and physical performance by aerobic capacity (VO2max), 30 second Sit-to-stand (STS) and Maximum Step Length (MSL). Linear mixed models were used in analyses. Results. One hundred and thirty-four of 163 included participants (82%) completed both rehabilitation stays and returned questionaires at T2. The majority of completers were females (81%), breast cancer survivors (60%), highly educated and with mean age of 52.8 years (SD of 8.1). Participants had higher level of symptoms and fatigue and lower functioning at admission compared to a Norwegian reference population. However, they reported higher physical exercise level and 47% reported improved work status from T0 to T2. Symptoms and functioning, fatigue, physical exercise and physical performance improved significantly from T0 to T1 and were maintained at T2. Conclusions. The rehabilitation program was feasible and symptoms and functioning normalized following rehabilitation. The program mainly recruited well-educated breast cancer survivors, reporting relative high level of physical exercise. More focus should be put on recruiting and selecting those who need comprehensive inpatient rehabilitation and also compare the effects of inpatient with outpatient rehabilitation programs.
The complexity of curative cancer treatment has increased and patients often receive multimodal treatment including surgery, radiotherapy and chemotherapy resulting in increased survival [Citation1]. A significant number of cancer survivors experience negative effects of the disease and the treatment on functioning, which may persist many years after cancer treatment has ended [Citation2]. As many as 63% of Norwegian cancer survivors have reported need for at least one rehabilitation service two to three years after diagnosis [Citation3]. Forty-three percent of the survivors reported need for physical therapy, followed by physical training (34%) and psychological counselling (27%) [Citation3].
Long-term effects of the cancer disease and treatment may cause impaired ability to stay in work even several years after end of treatment, and a meta-analysis showed that cancer survivors are more likely to be unemployed than healthy controls [Citation4]. In order to go back to normal life as soon as possible, different rehabilitation programs aiming to improve functioning and work ability have been proposed.
Complaints such as fatigue and other symptoms lead to lower quality of life, reduced physical activity and reduced functioning in activities of daily living during and after cancer treatment. A recent review of studies published the last two decades concluded that physical exercise is the most effective single intervention for decreasing fatigue and improving physical functioning [Citation5]. Furthermore, a Cochrane review concluded that multidisciplinary interventions combining physical training, patient education and counselling resulted in higher return to work rates compared to care as usual [Citation6]. Assessment of the effect of multidisciplinary rehabilitation has mainly been based on outpatient programs. Studies regarding inpatient programs for cancer patients are few, with significant heterogeneity in terms of design, patient characteristics, duration and content of the rehabilitation program. A recent published randomized study comparing a six-days in-patient psychosocial rehabilitation course with a light-to-moderate physical exercise component for cancer patients compared to usual care did not significantly relieve distress or improve well-being [Citation7]. In contrast, a multidisciplinary inpatients program with a pre-post design including physical exercise and education of three to four weeks duration among breast cancer patients showed promising improvements in several domains of health-related quality of life [Citation8]. In Norway, it has been a tradition to offer inpatient rehabilitation programs for patients with different chronic diseases such as chronic obstructive lung disease and cardiac diseases. In order to plan for the best future rehabilitation program for cancer survivors, better knowledge about the organization of cancer rehabilitation services is warranted, and thus the effect of inpatient rehabilitation programs should be explored.
Rehabilitation programs should be offered cancer survivors who have need for support to regain function. A recent cross-sectional survey found that living alone, having changed employment status due to cancer, receiving or have received chemotherapy and co-morbidities were factors associated with unmet needs of rehabilitation services [Citation3]. Furthermore, breast cancer survivors and those with high educational level more frequently report the need for physical training than other cancer survivors [Citation9].
A systematic review of rates of adherence and completion of exercise programs offered to cancer survivors show that about two-thirds of patients invited to undertake a program of therapeutic exercise program accept it, but only about half of those offered a program completed it [Citation10]. However, to our knowledge, no earlier study has addressed the characteristics, the adherence- and drop out rate among the participants attending inpatient and multidisciplinary rehabilitation programs for cancer survivors.
In 2008, and funded by the Norwegian goverment initiated program “Fast – Return to Work (Fast – RTW)”, the University Hospital in Trondheim in collaboration with a rehabilitation center in Norway started an inpatient rehabilitation program for cancer patients. The aim with this project was to systematically collect data among those who participated and examine feasibility and changes in work status, health related quality of life and fatigue from start of the program to immediately after the end of the program and at six months follow-up.
In the present study the aim was to examine patient characteristics, feasibility, level of symptoms and functioning at admission and change in symptoms, functioning and work status of a 3 + 1 week multidisciplinary inpatient cancer rehabilitation program. The following research questions were addressed: What characterizes the cancer survivors referred to the inpatient rehabilitation program (patient characteristics)? What is the level of HRQoL, fatigue and physical exercise at admission to the program compared to the age and gender matched Norwegian reference population (patient characteristics)? Who refers the cancer survivors (feasibility) and at what stage in the disease trajectory are they (patient characteristics)? How many cancer survivors complete the rehabilitation program (adherence) and what is the drop out rate (feasibility)? The second aim was to assess changes in symptoms and functioning, physical fatigue, level of physical exercise, physical performance and work status from admittance of the primary stay to the follow-up stay and six months later.
We hypothesized that at admittance, the cancer survivors have lower level of functioning and physical exercise, more symptoms and higher level of fatigue compared to the general population. We also hypothesized that these outcomes improved after rehabilitation and were maintained at six months after follow-up stay.
Material and methods
Study design and recruitment procedures
This was an open prospective intervention study among participants with different cancer diagnoses attending a new inpatient cancer rehabilitation program. All participants attending the cancer rehabilitation program were asked to participate in the period the pilot study was in progress, i.e. the inclusion criteria for inclusion in the study and for a stay at the center were the same. The patients were recruited by primary care physicians or from cancer departments at hospitals in the central part of Norway. Additionally, The Norwegian Labor and Welfare Administration (NAV) (http://www.nav.no/) also referred persons to the program. NAV is a public authority that is concerned with work-oriented initiatives and is a service aiming to get individuals on sick leave back to work. The new rehabilitation program was announced for doctors and nurses at the different cancer hospital departments through monthly information meetings, by written information and short features on the television screens in general practitioners’ waiting room. The study period was from August 2008 to October 2010.
The inclusion criteria were as follows: 1) having any cancer diagnosis; 2) being within employment age (18–67 years); 3) being employed and on sick leave or at self-defined risk of sick leave at the start of the program; 4) having completed primary cancer treatment (except for hormone therapy) and; 5) having Karnofsky`s performance status (KPS) of 70 and above [Citation11]. Exclusion criteria were alcohol- or drug abuse and mental impairment.
Assessments were performed at the start of the primary stay (T0), at arrival of the follow-up stay 8–12 weeks later (T1) and six months after the end of the follow-up stay (T2, mailed questionnaires). Demographic data were collected directly from the participants and medical data and co morbidities from the participants’ medical records and through information from the cancer survivor collected by the doctor.
The intervention
The inpatient cancer rehabilitation program took place at Røros Rehabilitation Centre located in the middle part of Norway. The participants attended a three weeks (15 days) primary stay and a one week (five days) follow-up stay eight to 12 weeks after the primary stay. On the weekends, the participants were encouraged to stay at the rehabilitation center and to be physically active. However, there was no scheduled program on the weekends and some participants preferred to travel home. The participants were enrolled in groups of 10 to 15 persons of both genders. At arrival all participants had a consultation with a nurse or a doctor where the participants set individual goals for their rehabilitation based on their self-defined needs. The goal combined with the initial assessments formed the basis for the specific content of their stay. All participants attended a common program consisting of physical training, patient education with group discussions using a cognitive approach focusing on change in dysfunctional thinking, behavior and emotional responses (Supplementary Table I, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.699684). Topics for the patient education were: 1) cancer disease, treatments and short- and long-term side-effects; 2) physical training; 3) nutrition; 4) the consequences of cancer disease on economy and work situation; 5) partnership; 6) psychological reactions and distress management and; 7) coping strategies related to living with cancer. The nutrition and diet education had a theoretical part complemented with a practical part in the kitchen. Patient education of various topics was run as lectures with group discussions lead by oncologists/physicians, physiotherapists/sport instructors, dieticians, social workers, sexual therapists/nurses and psychologists. Individual consultations were provided on request. In addition, as peers the participants discussed challenges during the stay.
The main goal of the physical training sessions was to improve aerobic capacity and stimulate to regular physical training. Physical training was performed twice a day with sessions lasting from 60 to 120 minutes, consisting of different indoors- and outdoors activities (see Appendix for full list of activities and detailed time schedule). During the physical training sessions, the participants were encouraged to push themselves to perceived intensities of 3–4 (“moderate intensity”, i.e. talking pace) on the modified Borg CR10 scale [Citation12] at least twice a week. For interval training, also at least twice a week, the perceived intensity was recommended to be 5–7, (“strong intensity” to “very strong intensity”, i.e. difficult to speak long sentences). The participants were advised to continue exercising between the primary- and follow-up stay.
The total time spent on physical training, patient educations and group discussions for each participant was approximately 100 hours. The lectures accounted for about 15 hours, the group discussions 25 hours, and the physical training 60 hours of the total active hours.
Health related quality of life (HRQoL) (EORTC QLQ-C30)
Physical-, physical role-, emotional-, cognitive-, and social functioning, symptoms (fatigue, nausea and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea), financial difficulties and global health status were assessed by scales from the cancer specific health related quality of life questionnaire European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) [Citation13]. The raw scores were linearly transformed to 0–100 scales [Citation13]. Higher scores represent better functioning. A difference of 10 points or more is considered as clinically relevant [Citation14]. Data from a randomly selected sample of 1965 Norwegians between 19 and 93 years of age drawn from the National Register were used as references/norms [Citation15]. The compliance rate was 68% including all follow-ups, the mean age 47.4 years, 48% were females, and 26% had university education.
Fatigue
Fatigue was assessed by the Norwegian version of the Fatigue Questionnaire (FQ) [Citation16]. FQ contains 11 items, giving a total fatigue score (all items, 0–33 scale) that is further divided into physical fatigue (seven items, 0–21 scale) and mental fatigue (four items, 0–12 scale). Higher scores indicate more fatigue. Data from a random sample of 2323 Norwegians drawn from the National Register was used as reference data. The response rate was 67%, the age range 19–80 years, mean age 44.9 (SD 16.5) years, 9% were females, and 28% had university education [Citation17].
Level of physical exercise
Physical exercise was assessed by the The Nord-Trøndelag Health Study Physical Activity Questionnaire (HUNT 1 PA-Q) developed as part of a Norwegian longitudinal population based health survey (http://www.ntnu.no/hunt/english) [Citation18], comprising one item on frequency, one on duration and one on intensity of physical exercise the last seven days. Physical exercise is addressed as; “for example going for walks, skiing, swimming or training/sport”. An index score was calculated based on the product of frequency, duration and intensity, giving a range from 0 (lowest) to 15 (highest) [Citation18]. The questionnaire has demonstrated acceptable test-retest reliability in adult males [Citation18]. Data from a random sample of 35 649 inhabitants from the HUNT 3 study (http://www.ntnu.no/hunt/english) [Citation19] were used as reference data. The response rate was 54%, the age range 19–92 years, mean age 53.4 (SD 14.8) years, 57% were females, and 26% had college/university education.
Work status
A single question about current work status was reported by the participants: “What is your present work situation?” The response alternatives were; 1) full-time work (%); 2) part-time work (%) and sick leave (%); 3) sick leave (100%); and 4) disabled or on rehabilitation benefit. Changes in work status were calculated by comparing work status at T0 and T2, with possibilities of: 1) decreased work status (a change from full- or part-time work status to sick leave or to a higher percent sick leave); 2) no change in work status (unchanged work status, sick leave or disablement/rehabilitation benefit); or 3) improved work status (decrease in proportion of sick leave, or from disablement/rehabilitation benefit to full- or part-time work status).
Physical performance tests
Aerobic capacity was assessed as VO2max (ml/kg/min) on a continuous stairway treadmill (Woodway PPS 55 med-I), 10 minutes test protocol using a Jaeger Oxycon Delta test instrument. The speed was constant and inclination of treadmill-ramp gradually increasing with 2% every minute up to exhaustion.
A repeated Sit-to-stand (STS) test was used as an indicator of lower body strength [Citation20]. It records how many times a patient manages to rise from sitting on a chair (43 cm seat height and without armrests) to full stand and return back to initial seated position within 30 seconds. Arms were crossed and held against the chest. The test has previously been tested in adults over 60 years of age with test-retest intraclass correlation of 0.84 for males and 0.92 for females [Citation20].
The Maximum Step Length (MSL) test was used to assess functional balance and strength [Citation21]. The test assesses maximal forward step length and was performed standing with parallel legs behind a marked line. The participants were told to take as long as possible step forward with one leg and the other leg planted, and in one step return to the initial position. The same optional leg was used each time. The procedure was repeated five times and the mean of the last three trials measured from toe to toe (cm) used as test variable. The test has previously been found to be a reliable and valid test in older females [Citation21].
Statistical methods
Statistical analyses were conducted using the software package SPSS, version 17.0 for Windows (SPSS, Inc., Chicago, Illinois, USA). Differences between completers and drop outs were tested by Fisher`s exact test, Mann-Whitney U-tests and Independent sample t-tests. Results are presented as means and 95% confidence intervals (CI). Norwegian reference data for the EORTC QLQ-C30 [Citation15], the FQ [Citation17], and HUNT 1 PA-Q [Citation18] were weighted according to the gender- and age distribution of the study sample of cancer survivors, using linear mixed models. Analyses of change (time effect) were assessed by linear mixed models using all available data for each subject. Mixed models are not affected by randomly missing data [Citation22]. For variables with significant time effect we performed post hoc tests of changes between T0 and T1 and between T0 and T2. Data on change are presented for main effect of time and effect of time. A significance level of p < 0.05 was used in all analyses and all tests were two-tailed.
Ethics
The study was conducted according to the guidelines of the Helsinki Declaration. Written informed consent was obtained from all patients prior to participation and the study was approved by The Norwegian Data Inspectorate Board and the Regional Committee for Medical and Health Research Ethics in Central Norway. Participation in the study was voluntary and did not affect the rehabilitation services.
Results
Completion rate
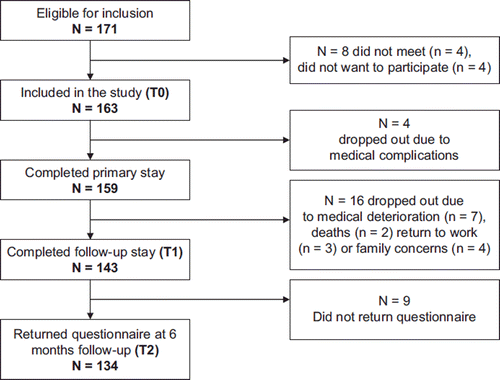
Hundred and seventy-one cancer survivors were admitted to the cancer rehabilitation program, of whom four did not meet and four did not want to participate in the study (). Thus, 163 participants were included at the start of the primary stay. Four dropped out during the primary stay due to medical complications, and 16 did not meet at follow-up stay due to the medical situation (n = 7), death (n = 2), return to work (n = 3) or family and social concerns (n = 4). Two died in the period between primary- and follow-up stay and two in the period between secondary stay and six months follow-up. Finally, 82% (n = 134) of the included participants completed the assessments at T0, T1 and T2.
Characteristics of the participants
shows demographics and medical characteristics for included participants (n = 163), completers and drop outs. A significantly higher portion of the drop outs were males (p = 0.048), more of the drop outs had high school as the highest educational level (p = 0.015), on disablement- or rehabilitation benefit (p = 0.017), a higher portion were smokers (p < 0.003), and significantly less of the drop outs had received previous hormonal treatment (p = 0.011). However, the drop outs reported lower mental fatigue compared to the completers (5.2 vs. 6.2 for the completers, p = 0.022).
Table I. Sample characteristics for completers and drop outs.
Forty-two percent of the included participants (n = 163) were referred from NAV, 34% from family physicians and 24% from hospital physicians. Mean age was 52.5 ± 8.1 years, ranging from 25–66 years, 77% were females, 56% had breast cancer, 34% had college/university education ≥ 4 years, mean KPS was 87 ± 12, and mean BMI 25.7 ± 4.2 kg/m2. Median time since cancer diagnosis was 0.9 years and 52% had been diagnosed with cancer within last year, 17% between one and two years earlier and 31% more than two years earlier. Twenty-eight percent of the completers had relapse of cancer and/or metastasis.
Levels of HRQoL, fatigue and physical exercise compared to reference data
Compared to an age and gender matched healthy Norwegian reference population, the participants had on admission significantly lower HRQoL, with scores between 13.0 and 37.1 points lower on the functional scales, between 3.4 and 22.5 higher on the symptom scales, 14.8 lower on the global health status, and with scores 5.5 and 1.8 points higher on respectively physical- and mental fatigue (). Self-reported level of physical exercise was however 1.5 points higher compared to an age and gender matched Norwegian reference data and remained higher at all three assessment points. HRQoL normalized during the follow-up, while fatigue was normalized already at T1.
Table II. Outcome scores at primary stay (T0), follow-up stay (T1) and at 6 months (T2) with p-values of change. Data at T0 are compared to reference data for age and gender matched Norwegian general populations.
Changes in self reported outcomes
Throughout the study period all functions improved, demonstrated by a significant main effect of time (p ≤ 0.014), except for financial impact (p = 0.35). There were significant changes in all symptoms and functioning scores, fatigue and level of physical exercise from T0 to T1 and from T0 to T2 (). PF increased from 74.3 at T0 to 83.6 at T1 (change of 9.3 points) and to 86.4 at T2 (change of 12.1 point) (p < 0.001). EF increased from 68.2 at T0 to 81.6 at T1 (change of 13.4 points) and to 79.9 at T2 (change of 11.7 points) (). Physical fatigue decreased from 13.6 at T0 to 8.1 at T1 (change of 5.5 points) and to 9.3 at T2 (change of 4.3 points) (p < 0.001). The remaining HRQoL-scores from the EORTC and physical, mental and total fatigue (FQ) are reported in . Level of physical exercise increased from 3.6 at T0 to 5.7 at T1 (increase of 2.1 points) and to 4.9 at T2 (increase of 1.3) (p < 0.001).
Physical performance tests
The level of VO2max increased with 2.5 ml/kg/min from T0 to T1 (from 31.3 to 33.8 ml/kg/min) (). The number of STS repetitions increased with 4.2 (from 18.2 to 22.4), and MSL with 7.7 cm (from 109.7 to 117.4 cm). All improvements were significant (p < 0.001).
Table III. Physical performance test scores at primary stay (T0) and follow-up stay (T1) with p-values of change.
Change in work status
Forty-seven percent of the participants reported improved work status from T0 to T2 (). At T2, 31% reported full-time work (17% at T0), 38% part-time work (17% at T0), 9% to be on sick leave (54% at T0) and 15% to be disabled or on rehabilitation benefit (11% at T0).
Table IV. Change in working status from T0 to T2.
Discussion
This study assessed patient characteristics of those attending a comprehensive program for cancer survivors, adherence and change in outcomes following a newly developed inpatient rehabilitation program with particular focus on physical training. The majority of the participants recruited were females, well-educated and breast cancer survivors. The high compliance rate indicate that the program was feasible. Patient-reported HRQoL outcomes were lower and fatigue and level of physical exercise higher compared to the age and gender matched Norwegian reference data at arrival. Forty-seven percent of the participants reported improved work status at follow-up nine months after admission, In addition, the HRQoL outcomes, fatigue, level of physical exercise and physical performance improved and were comparable to a Norwegian reference population, and for physical exercise, even higher than in a Norwegian reference population.
To our knowledge, this is the first study focusing on the patient characteristics and participation rate of an inpatient rehabilitation program for cancer survivors. The strengths of the study were the high compliance to the program and the six month follow-up. Compliance was comparable to other rehabilitation studies focusing on exercise interventions in cancer survivors [Citation10] and also to the response rate at six months follow-up [Citation23].
On admission, the participants in our study reported considerably more fatigue and had lower levels of functioning and more symptoms compared to the Norwegian reference populations [Citation15,Citation17]. This indicates that the program has attracted participants with reduced general functioning and with symptoms after cancer treatment and who experienced need for rehabilitation at the time of inclusion. Six months after finished rehabilitation, the participants’ functioning, symptoms and level of fatigue were comparable to the age and gender matched Norwegian reference populations, meaning that the functions had recovered and the symptoms had diminished during the follow-up period. Our results are in line with an earlier study showing positive change in several HRQoL-scores after three to four weeks inpatient complex rehabilitation program for breast cancer patients that were maintained at follow-up three months later [Citation8]. However, contrary, recently published results from a six-day residential course did not relieve cancer patients` distress or improve their well-being [Citation7] compared to usual care. The reason for no effects following this six-day course could possibly be explained by the short duration of the program or the fact that the participants in this study reported relatively high scores on HRQoL at inclusion compared to the participants in our study [Citation7]. Future study should explore in detail which participants need inpatient cancer rehabilitation and compare duration and content of inpatient programs using a randomized design.
Based on previous findings, the changes in most of the patient-reported HRQoL outcomes can be regarded clinically significant [Citation14]. Also VO2max demonstrated a mean improvement of 2.5 ml/kg/min (mean improvement in VO2max of 7.5%), which is comparable to percentage changes in physical capacity in healthy moderately trained males following high intensity interval training of three days a week over eight weeks (mean improvement in VO2max of 7.2%) [Citation24]. Thus, it is possible that the multidimensional rehabilitation program consisting of education as well as physical training was effective in improving functioning and symptoms in participants who had lower functioning and more symptoms and fatigue than the age and gender matched general populations. However, due to the lack of a control group we cannot conclude whether these improvements are merely an effect of time and the natural course of regaining normality after terminating cancer treatment.
Nearly half of the participants in the present study reported improved work status six months after the rehabilitation period. Cancer survivors are at increased risk of sick leave [Citation4] and studies have found multidisciplinary rehabilitation interventions including physical, psychological and vocational components to lead to higher return-to-work rates after cancer disease than care as usual [Citation5]. A prospective randomized control study on multidisciplinary outpatient rehabilitation found no differences on work status up to one year follow-up between the intervention and the control group, but however a strong time effect on return-to-work in both groups [Citation25]. Thus, we cannot conclude whether the improved work status among participants in our study represent the natural course of improved work ability after treatment.
The participants recruited to this inpatient rehabilitation program was self-selected and consisted of a heterogenous group of cancer survivors in terms of cancer diagnosis, cancer stage, time since diagnosis and cancer treatment, consequently a sample of convenience. The program seems to have attracted females, well-educated and breast cancer survivors reporting to be more physically active than the general population. In addition, a significantly higher proportion of the drop outs were males, had high school as the highest educational level, more were on disablement- or rehabilitation benefit and more were smokers indicating that the drop outs probably had a lower socio-economic status than those completing the rehabilitation. Thus, the sample of convenience and the skewed distribution concerning diagnosis and socio-economic status limits the possibility to generalize our results. However, this skewed distribution of participants may mirror the real life situation and those recruited may possibly be representative for those that can be reached and who are offered a rehabilitation stay.
There are several possible explanations for skewed distribution of the sample. The rehabilitation program emphasized physical training which may have attracted well-educated, physically active applicants motivated for physical training. In addition, the majority of this sample was recruited from NAV with an aim of improving employment status. The lower referral rate from family- and hospital physicians may be explained by lack of knowledge about the rehabilitation program or that they believed that the program was not profitable for their patients (gate-keeping). Finally, breast cancer survivors are more likely to report need for physical therapy and supportive care sessions than patients with other diagnoses [Citation3]. Whether the participants had knowledge about the inpatient rehabilitation program and felt need for rehabilitation or whether the physicians advised them to apply is not known.
This 3 + 1 weeks program is quite resource demanding and a shorter length of the program, e.g. a 2 + 1 week program, should be tested to explore if the changes are of the same order of magnitude. A follow-up week is organized to be able to respond to questions that may come after coming home, to provide security and to motivate to training between primary and follow-up stay.
Inpatient rehabilitation is expensive and should be offered cancer survivors in particular need of comprehensive rehabilitation. This include cancer survivors who live in areas where there are no rehabilitation offers in their municipality of residency, or who have complex problems and are suffering from nutrition problems and other severe symptoms like fatigue and pain. In our study participants with lower educational status, on disablement- or rehabilitation benefit (from lower social classes) and males were more likely to drop out of the inpatient rehabilitation program. The non-completers might represent a group that is hard to motivate for lifestyle change and the skewed distribution of the sample may represent a selection bias resulting in a higher percent of improved work status than in real life. If this is a problem in relation to inpatient programs or whether this phenomenon also is present in outpatient programs remains to be answered and should be given priority in future research. However, the drop out characteristics could also mirror the real life situation in that the drop outs have more of the risk factors associated with unstable employment.
The main question after termination of this study is whether the program has recruited cancer survivors with the greatest need for inpatient rehabilitation. Future studies should examine more closely who are in need of, and should be offered comprehensive inpatient rehabilitation and how to recruit these cancer survivors.
Conclusion
A majority of participants attending this newly developed two step comprehensive inpatient cancer rehabilitation program focusing on physical training were females, breast cancer survivors and with relatively high educational level. Furthermore, they reported more symptoms, impaired functioning, but higher level of physical exercise than the Norwegian reference population. There was a high compliance rate and following rehabilitation significant improvements in functioning, fatigue and physical performance were detected. However, we cannot conclude whether these improvements are an effect of the rehabilitation program or just the natural course of regaining normality after terminating cancer treatment. In the future, more focus should be put on recruiting and selecting those who need comprehensive inpatient rehabilitation and also compare the effects of inpatient with outpatient rehabilitation programs.
Table I
Download PDF (150.7 KB)Acknowledgements
We thank all the participants in this study who willingly filled in the questionnaires. Thanks to the Nord-Trøndelag Health Studies (The HUNT Study, including the HUNT 3 study) which is a collaboration between HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology, NTNU), Nord-Trøndelag County Council, and the Norwegian Institute of Public Health. We also want to thank Jon Håvard Loge and Marianne J. Hjermstad for the opportunity to analyze the Norwegian reference data of respectively level of physical exercise (HUNT 1 PA-Q), HRQoL (EORTC-QLQ C30) [Citation15] and fatigue (FQ) [Citation17]. This study was conducted in collaboration with Røros Rehabilitation, the Norwegian University of Science and Technology and St Olav University Hospital in Trondheim. The project was supported by grants from The Norwegian Cancer Society. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Juvet L, Elvsaas I, Leivseth G, Anker G, Bertheussen GF, Falkmer U, . Rehabilitation of breast cancer patients. Oslo: Norwegian Knowledge Centre for the Health Services (Nasjonalt kunnskapssenter for helsetjenesten); 2009.
- Smith T, Stein KD, Mehta CC, Kaw C, Kepner JL, Buskirk T, . The rationale, design, and implementation of the American Cancer Society’s studies of cancer survivors. Cancer 2007;109:1–12.
- Thorsen L, Gjerset GM, Loge JH, Kiserud CE, Skovlund E, Flotten T, . Cancer patients’ needs for rehabilitation services. Acta Oncol 2011;50:212–22.
- de Boer AG, Taskila T, Ojajarvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: A meta-analysis and meta-regression. JAMA 2009;301:753–62.
- Richardson A, Addington-Hall J, Amir Z, Foster C, Stark D, Armes J, . Knowledge, ignorance and priorities for research in key areas of cancer survivorship: Findings from a scoping review. Br J Cancer 2011;105(Suppl 1): S82–94.
- de Boer AG, Taskila T, Tamminga SJ, Frings-Dresen MH, Feuerstein M, Verbeek JH. Interventions to enhance return-to-work for cancer patients. Cochrane Database Syst Rev 2011;(2):CD007569.
- Rottmann N, Dalton SO, Bidstrup PE, Wurtzen H, Hoybye MT, Ross L, . No improvement in distress and quality of life following psychosocial cancer rehabilitation. A randomised trial. Psychooncology Epub2011 Feb 8.
- Heim ME, Kunert S, Ozkan I. Effects of inpatient rehabilitation on health-related quality of life in breast cancer patients. Onkologie 2001;24:268–72.
- Gjerset GM, Fossa SD, Courneya KS, Skovlund E, Jacobsen AB, Thorsen L. Interest and preferences for exercise counselling and programming among Norwegian cancer survivors. Eur J Cancer Care (Engl) 2011;20:96–105.
- Maddocks M, Mockett S, Wilcock A. Is exercise an acceptable and practical therapy for people with or cured of cancer? A systematic review. Cancer Treat Rev 2009;35:383–90.
- Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 1980;45:2220–4.
- Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health 1990;16(Suppl 1):55–8.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Health- related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: the QLQ = C30 (+ 3). J Clin Oncol 1998;16:1188–96.
- Chalder T, Berelowitz G, Pawlikowska T, Watts L, Wessely S, Wright D, . Development of a fatigue scale. J Psychosom Res 1993;37:147–53.
- Loge JH, Ekeberg O, Kaasa S. Fatigue in the general Norwegian population: Normative data and associations. J Psychosom Res 1998;45(1 Spec No):53–65.
- Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trondelag Health Study: HUNT 1 Scand J Public Health 2008;36:52–61.
- The Nord-Trøndelag Health Study (HUNT). JAGS 1, 1–2. 2010. Ref Type: Internet Communication
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113–9.
- Medell JL, Alexander NB. A clinical measure of maximal and rapid stepping in older women. J Gerontol A Biol Sci Med Sci 2000;55:M429–33.
- Gueorguieva R, Krystal JH. Move over ANOVA: Progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 2004;61:310–7.
- Kaasa S, Hjermstad MJ, Jordhoy MS, Wisloff F, Loge JH. Compliance in quality of life data: A Norwegian experience. Stat Med 1998;17:623–32.
- Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, . Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc 2007;39:665–71.
- Berglund G, Bolund C, Gustafsson UL, Sjoden PO. One-year follow-up of the ‘Starting Again’ group rehabilitation programme for cancer patients. Eur J Cancer 1994;30A:1744–51.