Abstract
Background. Newer studies raise concern that adjuvant anthracycline treatment for breast cancer (BC) causes long-term heart damage. We aimed to examine whether heart failure or impairment could be demonstrated several years after low-dose epirubicin-based adjuvant treatment. Material and methods. The study-population was a historical cohort comprising 980 women who were randomized to receive one of two adjuvant regimens for treatment for BC: 7–9 cycles of cyclophosphamide-epirubicin-5-fluorouracil [CEF (600 + 60 + 600 mg/m2)] or cyclophosphamide-methotrexate-5- fluorouracil [CMF (600 + 40 + 600 mg/m2)]. We collected information in national registries of death and diagnoses and a sample of 77 survivors was examined with tissue-Doppler imaging (TDI), echocardiography, radionuclide ventriculography and N-terminal-pro-B-type-natriuretic peptide (NT-proBNP), an established marker for heart failure. Results and conclusion. Median follow-up was 12 years (39 days–20 years). Fifty-one percent had died. Incidence of CHF was 2.6/1000/year and equal in the treatment groups. In the sample, individuals who had received CEF showed no cardiac impairment when compared to individuals who received CMF. NT-proBNP-levels were within normal limits but higher in the CEF-group than in the CMF-group (confidence limits 105–226%, p = 0.03). Results of our study seem reassuring regarding the long-term risk of cardiotoxicity following low-dose adjuvant epirubicin treatment. However, larger, longitudinal studies are needed to establish the clinical implications.
Several drugs used in the treatment of breast cancer have the propensity to cause heart damage but best known is the cardiotoxicity complicating treatment with anthracyclines (AC). Toxicity induced by AC is probably multifactorial but oxidative stress causing damage to the myocardium is believed to have a central part [Citation1]. The cardiotoxicity is recognized as chronic heart failure (CHF) developing months to years after ended treatment and affecting up to 20–50% [Citation2]. The risk of cardiotoxicity is dose-related and promoted by several risk-factors including heart disease, advanced age and concomitant treatment with other cardiotoxic drugs [Citation3]. Traditionally, the preferred anthracycline used for treatment of breast cancer in USA is doxorubicin, whereas in Europe the derivative epirubicin is used. At equimolar doses epirubicin is as effective as doxorubicin but hampered by less toxicities [Citation4]. The equitoxic dose-ratio regarding cardiotoxicity is approximately 2:1 allowing larger doses of epirubicin to be used [Citation5].
However, the risk of serious cardiotoxicity has restricted the cumulative dose used, and therapy with AC has not been recommended for elderly individuals even though a meta-analysis indicates that individual risk factors rather than age should be considered [Citation6]. Until recently low doses of AC as used in adjuvant treatment regimens have been assumed to be safe without any considerable risk of long-term cardiac sequelae. New studies, on the other hand, have given concern that even low-dose treatment carries a risk of deterioration of the heart function many years after exposure [Citation7–9]. Adjuvant regimens substituting AC with methotrexate have been regarded as less cardiotoxic even though not favored by disease-free survival [Citation10]. Some studies indicate that individuals with breast cancer belonging to certain high-risk subgroups might benefit from increased doses of AC, and other studies suggest that patients with advanced age might also take advantage of adjuvant chemotherapy [Citation11]. Before implementing new adjuvant AC treatment regimens for these entities it is important to evaluate the risk of chronic cardiac damage associated with current treatment regimens.
Various techniques have been applied in the monitoring of the cardiac function during chemotherapy. Traditionally this has included measurements of the left ventricular ejection fraction (EF) assessed with radio-nuclide ventriculography (MUGA) even though some studies indicate that changes in EF-measurements are inadequate to predict later deterioration of the cardiac function [Citation12–14]. Diastolic measurements derived from MUGA have been suggested as alternatives. However, in a recent study we found these variables added little information to conventional EF-measurements [Citation15].
Objective
In the present study of a historical cohort our aim was to examine if heart failure or impairment can be demonstrated several years after even low-dose adjuvant epirubicin-treatment for breast cancer.
Material and methods
The publication is based on registry studies of a historical cohort (). A sample of the survivors from the cohort (sample) was further examined using different modalities to determine heart function status at late follow-up: Tissue-Doppler echocardiography, MUGA and plasma N-terminal-pro-B-type natriuretic peptide (NT-proBNP).
Figure 1. The historical cohort used in this study represents the Danish participants from a multinational randomized trial [Citation26].
![Figure 1. The historical cohort used in this study represents the Danish participants from a multinational randomized trial [Citation26].](/cms/asset/fd06de39-ba11-472f-923e-b93c9482b932/ionc_a_702920_f0001_b.gif)
The study was accepted by the Scientific, Ethics Committees for the Capital Region and written informed consents were obtained from all participants.
Historical cohort
The historical cohort consisted of 980 Danish participants from a total of 1224 women () included between 1989 to 1998 in a multi-center, randomized, open-labeled phase 3 trial comparing two adjuvant chemotherapy regimens for breast cancer: CEF (cyclophosphamide + epirubicin + 5-fluorouracil) versus CMF (cyclophosphamide + methotrexate + 5-fluorouracil) [Citation10]. The trial was conducted by Danish Breast Cancer Cooperation Group (DBCG) and results were in favor of CEF-treatment in terms of the hazard ratio (HR) for disease-free survival (HR 0.84) and death (HR 0.79) [Citation10].
CEF and CMF treatments were administered every three weeks in seven to nine cycles, 600 + 60 + 600 mg/m2 or 600 + 40 + 600 mg/m2, respectively, per treatment cycle.
At inclusion, all patients had completely resected unilateral breast cancer and none had heart diseases contraindicating treatment with epirubicin.
Of 980 randomized participants in the cohort 18 ended up receiving neither CEF nor CMF. Of the remaining 962 individuals 46.5% (n = 447) were treated with CEF and 53.5% (n = 515) received CMF. Radiation therapy (RT) was given to 64% (n = 616). Mean age at treatment start was 48.3 ± 9.1 years in the CEF-group and 48.0 ± 8.9 years in the CMF-group. Follow-up times ranged from 39 days to 20 years (geometric mean 12 years) after randomization.
We used the Danish civil registration number (CPR) assigned to all Danish residents to link datasets. From the DBCG database we collected patient and tumor characteristics on therapies (radiation- and systemic therapy) and outcome. From the Danish National Board of Health (LPR) we collected information on diagnoses from hospital admissions and out-patient clinic visits, indicating ischemic heart disease (IHD) and CHF [Citation16].
Sample
A sample of surviving participants from the cohort was invited for further examinations. For logistical reasons only surviving patients from Copenhagen centers were invited.
From 120 invited persons 77 chose to participate and complete the study procedures (). The individuals in the sample were 33 from the CMF treatment group and 44 from the CEF group. Of these, 63% and 55%, respectively, received additional radiation therapy.
None of the examined patients had clinically significant valvular disease or clinical heart failure. All had normal sinus rhythm. Two individuals were treated for IHD and 10 for hypertension. Both conditions were evenly distributed between the treatment groups.
All individuals in the sample were examined using the following techniques:
Echocardiography. Echocardiographic recordings were done at the time of end-expiration with the subjects lying in left lateral decubitus position. Studies were stored for later analyses blinded for clinical data. In all examinations identical ultrasound units were used (GE Vingmed Vivid 7 with a 2.5 MHz probe) and second harmonic imaging was applied. ECHO-PAC PC ’08 software package was used for analysis of the echocardiographic studies. Recordings and subsequent analyses were made by a single investigator (JMA).
In the echocardiographic studies recordings were made in series of three consecutive beats. They included two-dimensional grey-tone images, M-mode, pulsed-wave Doppler (PWD), continuous-wave Doppler, color-Doppler and color tissue-Doppler imaging (TDI). TDI was obtained in apical 2- and 4-chamber views including anterior, posterior, lateral and septal sections of the mitral ring. The sector was minimized to achieve high temporal resolution with frame-rates exceeding 175 frames/s.
The following variables were collected based on the echocardiographic recordings: EF, mitral flow velocities E (early diastolic), A (late diastolic) and Edt (E deceleration time), Tei-index, flow propagation velocity (Vp) and mitral ring velocities E’ (early diastolic), A’ (late diastolic), S’ (Systolic) and peak systolic displacement. The tissue velocities were averaged between the four collection points.
Radio-nuclide ventriculography (MUGA). MUGA-examinations were performed after a standard protocol incorporating steady state, Tc-99m labeled HSA ventriculography with multiple gated ECG-triggered sampling in a left anterior oblique (LAO) view. Sampling with 32 frames per RR interval was used.
Examinations were analyzed three times by two experienced technicians and the final time-activity-curve was developed as the median of six analyses. EF, peak ejection rate (PER), time to peak ejection rate (TTPER), peak filling rate (PFR) and time to peak filling rate (TPFR) were calculated from the time-activity curve and its first derivative [Citation15].
N-terminal pro-BNP. Blood samples were collected after 15 min horizontal rest. The blood samples were immediately cooled to 4°C and centrifuged 10 min at 3000 rpm. Plasma was transferred to acrylic tubes and stored for a maximum of one month before analysis. A sensitive and specific immunoassay based on double-antibody sandwich technique was used for determination of plasma NT-proBNP according to the manufacturer's instructions (Immuno 2500, Siemens Healthcare diagnostics, Deerfield, IL, USA).
Statistical analyses
Continuous data are presented as mean ± standard deviation (SD) for normally distributed data, median and range for skewed distributions and as percentages for categorical data.
Values measured from CW, PW and TDI-traces, Vp and TAPSE were averaged over three consecutive beats before analysis. T-test was applied for comparison of groups and Levene's test was used to test equality of variances. NT-proBNP measurements had skewed distributions and were logarithmically transformed before analyses. Linear regression models with analyses for interactions were used for assessing the relation between variables. Pearson's test was applied for analysis of correlation between continuous, normally distributed data. For multiple comparisons the p-values were adjusted according to Bonferroni's method. In analysis of categorical register-data χ2-test and Fischers’ exact-test were used. Logrank test was applied for survival analysis. CHF-free survival was calculated as time from surgery to first diagnosis indicating CHF, with censoring for death and ischemic heart disease. IHD-free survival was calculated as time from surgery to first diagnosis indicating ischemic heart disease, when censoring for death.
All tests were two-sided and p-values below 0.05 were considered statistically significant.
The observed statistical power in the study exceeded 0.9 with a detection limit of 1 cm/s difference in tissue velocities E’ and S’ and α = 0.05.
Calculations were made with SAS and SPSS statistical software (SAS 9.1 for Windows and SPSS Statistics, version 18.0).
Results
Cohort
Among 980 participants in the original trial, 51% (n = 500) had died. Forty-three percent (n = 421) had recurrence of breast cancer. Ischemic heart disease (IHD) developed in 3.5% (n = 35) and 2.7% (n = 26) developed CHF. For the total population the incidence of heart failure was 2.5/1000/year at risk, including events occurring after development of ischemic heart disease. The incidence of IHD was 3.7/1000/year at risk. Development of IHD and CHF was more common in the elderly with a linear trend across age groups (p = 0.04 and 0.02, respectively).
Filtering cases not receiving adjuvant treatment and cases where IHD preceded or developed simultaneously with CHF left 1.0% (nCEF = 5, nCMF = 5) with heart failure as first diagnosis. There was no significant difference in the risk of primary CHF between the treatment groups (Logrank test, χ2 = 0.07, p = 0.93). There was also no difference when stratifying for radiation therapy (RT) (Logrank test, χ2 = 0.01, p = 0.976). The risk of IHD did not differ between individuals receiving RT and those not receiving RT (Logrank test, χ = 0.527, p = 0.468). Stratifying for adjuvant treatment group did not reveal any differences (Logrank test, χ2 = 0.475, p = 0.49).
Sample
Echocardiography and MUGA. There were no significant deviations between the two treatment groups in several diastolic and systolic parameters of right and left ventricular function from TDI, conventional echocardiography and MUGA (). Analysis of variances showed correlation to age for several variables but no effects from treatment regimen, radiation therapy or their interaction.
Table I. Sample.
Agreement between EF acquired from MUGA and echocardiography was ± 16%. No correlation was found between diastolic filling variables in MUGA and TDI (PFR and E’) (r = −0.12, p = 0.321). Confining to echocardiographic variables there was a significant correlation between EF and S’ (p = 0.002).
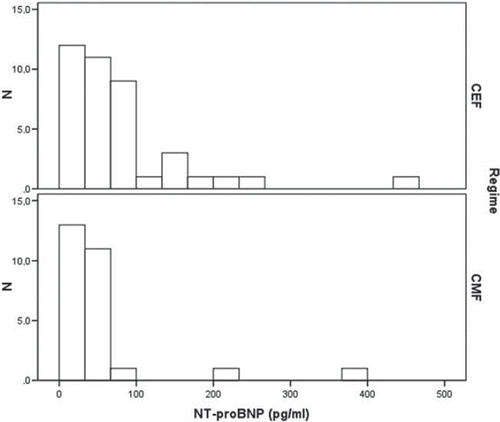
NT-proBNP. NT-proBNP values in the CEF treatment-group were statistically significantly larger with geometric means for CEF- and CMF-groups 59 (range 20–456) pg/ml and 38 (range 20–371) pg/ml, respectively, [Confidence limits (CL) 105–226%, p = 0.03] (). There was no interaction from radiation therapy. Systolic echocardiographic measurements, S’ and EF, correlated significantly to LogNT-proBNP (p = 0.006 and p = 0.008, respectively), EF-measurements from MUGA only marginally (p = 0.054).
Reference values provided by the manufacturer were wide with 95%-percentile values 110 pg/ml for individuals < 75 years and 589 pg/ml for individuals ≥ 75 years. Since NT-proBNP levels in normal individuals are particularly age and sex specific we used as reference values 97.5% percentiles from a large study of blood donors [Citation17]. This study used a similar analysis (Elecsys) expected to have essentially same reference range (DPC, 2006).
In the sample we found only abnormal measurements from two individuals aged 59 and 52 years who presented with elevated NT-proBNP, 456 pg/ml and 371 pg/ml, respectively.
One subject belonged to the CEF treatment-group, the other to the CMF-group. Neither had received RT. Both had IHD with normal EF values but tissue-Doppler velocities in the lowest 5–10% of the population and mitral flow velocities indicating degrees of diastolic dysfunction.
Discussion
Heart failure complicating cancer treatment with anthracyclines is well known following high cumulative doses but there is controversy about the risks associated with lower doses as used in adjuvant treatment for breast cancer. Some authors using different techniques have described short-term changes in the heart function following low-dose treatments [Citation18,Citation19]. However, we failed to demonstrate significant deterioration in multiple parameters from conventional and tissue-Doppler echocardiography in a recent study [Citation20]. Still, there is concern of a possible long-term damage to the cardiac function and regimens with less cardiotoxic liposomal formulations or regimens not containing anthracyclines have been tested. In the present registry based study of the original cohort we compared heart function in individuals treated up to 20 years previously with an epirubicin containing CEF-regimen to the supposedly less cardiotoxic CMF-regimen. Accounting for the considerable mortality in the trial the incidences of congestive heart failure and of ischemic heart disease were similar to a background population and the data did not show an increased risk of heart failure in the CEF-group compared to the CMF-group [Citation21,Citation22].
Observational studies of anthracycline cancer treatment have led to adoption of upper limits for cumulative anthracycline dosing [Citation1]. Treatment with higher cumulative doses results in a dramatic increase in the risk for CHF [Citation23]. For epirubicin the accepted upper limit is approximately 950 mg/m2 whereas the CEF-treated patients in our cohort received a rather low cumulative dose, approximately 500 mg/m2. It is possible that a certain critical cumulative dose is necessary for the development of cardiotoxicity and one reason why we did not detect an excess of CHF- incidents in these patients could be that the cumulative dose used was below the critical level.
To investigate whether more sensitive parameters would indicate long-term damage we invited a sample of surviving participants for further examinations.
Myocardial velocities measured throughout the heart cycle with tissue-Doppler imaging (TDI) are sensitive parameters of the cardiac function. They are affected early during development of several diseases from hypertension to diabetes. Using several systolic and diastolic variables from tissue-Doppler, conventional echocardiography and MUGA no differences in the heart function between the treatment regimens emerged.
BNP (brain-type natriuretic peptide) is released from the ventricular myocardium in response to left ventricular overload. The inactive NT-proBNP is formed equimolar with BNP through cleavage of the prohormone proBNP and both are established markers of systolic heart failure and increase with severity of the deterioration of heart function. However, these hormone levels also contain information of diastolic dysfunction and prognosis regarding death, future development of heart failure and other cardiovascular events [Citation24]. In the sample only two individuals presented with elevated NT-proBNP. They both suffered from degrees of diastolic dysfunction ascribed to ischemic heart disease. Excluding the two outliers from analysis the levels of NT-proBNP in the treatment groups, with CEF being marginally higher, were in the normal range and well below suggested cut-off levels used for exclusion of CHF [Citation25]. Conversely, the risk of death and cardiovascular events have been demonstrated to increase over a range from low to high levels of NT-proBNP and therefore it cannot be excluded that the difference in NT-proBNP represents a slightly higher risk for future adverse cardiovascular events in the CEF-group [Citation8,Citation24,Citation25].
Strengths and limitations
Registered data on the historical cohort gave little information of traditional risk factors for ischemic heart disease and heart failure. However, the selective inclusion in the original trial of patients without known heart disease, the randomization and the possibility of 100% follow-up through national registries gave an opportunity for comparison of cardiac risk related to the two treatment regimens without many of the usual caveats associated with retrospective studies.
Diagnoses from the national registries give conservative estimates of incidence of heart failure and ischemic heart disease since only cases treated in hospitals and outpatient clinics are recorded. Besides, undiagnosed heart disease causing sudden cardiac death will also be missed.
Several previous studies have shown that radiotherapy of the chest increases the risk of developing ischemic heart disease but this could not be demonstrated in our study [Citation26]. However, we had no information of whether tumor was located on the left or right side of the chest. This could be important for the cardiac exposure to radiation and omitting side from analysis of radiation risk may have weakened the result. On the other hand, some authors have demonstrated increased risk of IHD even with right-sided radiation therapy [Citation27].
A major limitation of to the study is the available sample of only approximately 8% of the original population. The main problem is the risk of bias induced by the mortality among the participants in the original trial. Besides, the invitation to participate might predominantly be accepted by the more healthy survivors making estimates too optimistic. Furthermore, individuals may have received other treatments or developed conditions influencing the risk of heart disease. In this study we were unable to account for these factors.
Even though methotrexate is not recognized as an agent causing chronic cardiotoxicity a potential effect could mask epirubicin-related changes in the other treatment arm. Several anti-neoplastic drugs have the potential of causing cardiotoxicity, including cyclophosphamide and 5-fluorouracil but a possible effect would not bias the result since both treatment groups received the combination [Citation28].
Conclusion
In a registry-based study of a historical cohort of 980 patients we found that incidence of heart failure was not increased in the group of patients who had received low-dose adjuvant epirubicin treatment for breast cancer compared to a regimen containing methotrexate. In contrast to several previous studies we could not demonstrate increased risk of ischemic heart disease associated with radiation therapy.
In search for a subclinical deterioration a sample of 77 survivors from the cohort were invited for further examinations with tissue-Doppler imaging, traditional echocardiography and MUGA but no differences in heart function between the treatment groups emerged. Even though both groups had normal plasma-NTproBNP a marginally higher level in the epirubicin-treated patients could indicate a slightly higher risk for later cardiac events.
Risk for secondary heart disease is an important aspect when defining the best adjuvant treatment for primary breast cancer in a given patient. Within the limitations of our study the results give some reassurance regarding potential long-term cardiac complications associated with a standard adjuvant low-dose epirubicin regimen.
Large studies are necessary to firmly establish the risk of cardiac toxicity associated with cancer therapy and to generate recommendations. An international registry of cardiotoxic complications would offer important information.
Acknowledgements
DBCG assisted with registry data and statistical analyses. Departments of Cardiology, Oncology and Nuclear Medicine, Herlev Hospital contributed with facilities, technical equipment and logistic help. Financial support was given by Region Hovedstadens Forskningsfond, Forskningsrådet Herlev Hospital and Fonden til Fremme af Medicinsk Behandling af Cancer.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Appel JM, Nielsen D, Zerahn B, Jensen BV, Skagen K. Anthracycline-induced chronic cardiotoxicity and heart failure. Acta Oncol 2007; 46: 576–80.
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003; 97: 2869–79.
- Ryberg M, Nielsen D, Cortese G, Nielsen G, Skovsgaard T, Andersen PK. New insight into epirubicin cardiac toxicity: Competing risks analysis of 1097 breast cancer patients. J Natl Cancer Inst 2008; 100: 1058–67.
- Khasraw M, Bell R, Dang C. Epirubicin: Is it like doxorubicin in breast cancer?A clinical review. Breast 2012; 21: 142–9.
- Launchbury AP, Habboubi N. Epirubicin and doxorubicin: A comparison of their characteristics, therapeutic activity and toxicity. Cancer Treat Rev 1993; 19: 197–228.
- Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, . Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA 2005; 293: 1073–81.
- Ganame J, Claus P, Uyttebroeck A, Renard M, D’Hooge J, Bijnens B, . Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr 2007; 20: 1351–8.
- Feola M, Garrone O, Occelli M, Francini A, Biggi A, Visconti G, . Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: Effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol 2011; 148: 194–8.
- Bonneterre J, Roche H, Kerbrat P, Fumoleau P. http://intl-jco.ascopubs.org/content/22/15/3070.full - aff-1, Goudier M-J http://intl-jco.ascopubs.org/content/22/15/3070.full-aff-1, Fargeot P http://intl-jco.ascopubs.org/content/22/15/3070.full-aff-1, http://intl-jco.ascopubs.org/content/22/15/3070.full-aff-1, http://intl-jco.ascopubs.org/Philippe + Montcuquet&sortspec = date&submit = Submit . Long-term cardiac follow-up in relapse-free patients after six courses of fluorouracil, epirubicin, and cyclophosphamide, with either 50 or 100 mg of epirubicin, as adjuvant therapy for node-positive breast cancer: French adjuvant study group. J Clin Oncol 2004; 22: 3070–9.
- Ejlertsen B, Mouridsen HT, Jensen MB, Andersen J, Cold S, Edlund P, . Improved outcome from substituting methotrexate with epirubicin: Results from a randomised comparison of CMF versus CEF in patients with primary breast cancer. Eur J Cancer 2007; 43: 877–84.
- Bonneterre J, Roche H, Kerbrat P, Brémond A, Fumoleau P, Namer M, . Epirubicin increases long-term survival in adjuvant chemotherapy of patients with poor-prognosis, node-positive, early breast cancer: 10-year follow-up results of the French Adjuvant Study Group 05 randomized trial. J Clin Oncol 2005; 23: 2686–93.
- Belham M, Kruger A, Mepham S, Faganello G, Pritchard C. Monitoring left ventricular function in adults receiving anthracycline-containing chemotherapy. Eur J Heart Fail 2007; 9: 409–14.
- Hensley ML, Schuchter LM, Lindley C, Meropol NJ, Cohen GI, Broder G, . American Society of Clinical Oncology clinical practice guidelines for the use of chemotherapy and radiotherapy protectants. J Clin Oncol 1999; 17: 3333–55.
- Jensen BV, Skovsgaard T, Nielsen SL. Functional monitoring of anthracycline cardiotoxicity: A prospective, blinded, long-term observational study of outcome in 120 patients. Ann Oncol 2002; 13: 699–709.
- Appel JM, Jensen BV, Nielsen DL, Ryberg M, Zerahn B. Systolic versus diastolic cardiac function variables during epirubicin treatment for breast cancer. Int J Cardiovasc Imaging 2010; 26: 217–23.
- Andersen TF, Madsen M, Jorgensen J, Mellemkjoer L, Olsen JH. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull 1999; 46: 263–8.
- Hess G, Runkel S, Zdunek D, Hitzler WE. Reference interval determination for N-terminal-B-type natriuretic peptide (NT-proBNP): A study in blood donors. Clin Chim Acta 2005; 360: 187–93.
- Karakurt C, Kocak G, Ozgen U. Evaluation of the left ventricular function with tissue tracking and tissue Doppler echocardiography in pediatric malignancy survivors after anthracycline therapy. Echocardiography 2008; 25: 880–7.
- Mantovani G, Madeddu C, Cadeddu C, Dessi M, Piras A, Massa E, . Persistence, up to 18 months of follow-up, of epirubicin-induced myocardial dysfunction detected early by serial tissue Doppler echocardiography: Correlation with inflammatory and oxidative stress markers. Oncologist 2008; 13: 1296–305.
- Appel JM, Sogaard P, Mortensen CE, Skagen K, Nielsen DL. Tissue-Doppler assessment of cardiac left ventricular function during short-term adjuvant epirubicin therapy for breast cancer. J Am Soc Echocardiogr 2011; 24: 200–6.
- Nielsen OW, Raymond IE, Kirk V, Pedersen F, Bay-Nielsen M. [The epidemiology of heart failure from a Danish perspective]. Ugeskr Laeger 2004; 166: 243–7.
- Wang TJ, Larson MG, Levy D, Leip EP, Benjamin EJ, Wilson PW, . Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002; 90: 254–8.
- Von Hoff DD, Layard MW, Basa P, Davis HLJr, Von Hoff AL, Rozencweig M, . Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979; 91: 710–7.
- Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA 2005; 293: 1609–16.
- Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol 2007; 50: 2357–68.
- Heidenreich PA, Schnittger I, Strauss HW, Vagelos RH, Lee BK, Mariscal CS, . Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol 2007; 25: 43–9.
- Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, http://www.ncbi.nlm.nih.gov/pubmed?term=Taylor%20CW%5BAuthor%5D&cauthor=true&cauthor_uid=17341728 . Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99: 365–75.
- Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009; 53: 2231–47.