Genome wide association studies need validation
The unexplained variability in drug effects, desired and undesired alike, remains a challenge in medical oncology. Individualized treatment aims at eliminating both unnecessary adverse drug reactions and unnecessary treatment. Taxanes are of undisputed benefit in ovarian cancer and a wide range of other solid tumors [Citation1]. Serious and sometimes irreversible neuropathy and bone marrow suppression affects a significant proportion of patients causing treatment delay and decreased quality of life of the patients. A huge effort has been put in identifying single nucleotide polymorphisms (SNPs) as predictors of toxicity, but successes with clinical application are yet to be seen. The recent advances in cost effective high throughput genotyping has led to more frequent use of genome wide association studies (GWAS) instead of the hypothesis driven candidate gene studies. GWASs are performed without a hypothesis and while the method involves correction for multiple testing (Bonferroni or similar), a corresponding adjustment of effects is usually not implemented. It is therefore often necessary to validate the results in other cohorts and to perform functional studies in order to consolidate the findings [Citation2].
Recently, Schneider and coworkers presented the preliminary results of a GWAS performed in 2204 breast cancer patients of mixed ethnicity treated with weekly paclitaxel in a phase 3 trial [Citation3] (NCT00433511). The primary endpoint was time to grade 2–4 neuropathy (Common toxicity Criteria) and at the interim analysis 613 patients had grade 2–4 neuropathy (28%). Promising gene-variant candidates included RWDD3 (rs2296308) and TECTA (rs1829). RWDD3 showed an allele dose dependent association with time to neuropathy with a hazard ratio of 1.5 per allele; neuropathy frequencies were 27% (wildtype/wildtype), 40% (wt/variant) and 60% (var/var), respectively, p = 8.5 × 10−8. TECTA showed that homozygocity for the variant genotype was associated with a neuropathy hazard ratio of 2.07 compared to other genotypes, p = 3.15 × 10−7; neuropathy frequencies were 29%, 30% and 55% for wt/wt, wt/var and var/var, respectively.
The aim of this study was to replicate the reported association between the RWDD3 (rs2296308) and TECTA (rs1829) and time to neuropathy.
Patients and methods
We retrospectively included patients from three cohorts of Scandinavian women with ovarian cancer treated with paclitaxel 175 mg/m2, q3w and carboplatin AUC 5–6 mg*min/mL. The cohorts have previously been described in detail [Citation4–6]. Briefly, patients were included if DNA was available along with time to neuropathy data. The study was approved by ethics committees in Denmark and Sweden, and was carried out in accordance with the Helsinki declaration.
RWDD3 (rs2296308) and TECTA (rs1829) genotyping was performed using TaqMan® SNP genotyping assays (Applied Biosystems, Foster City, CA) on a StepOne Plus machine (Applied Biosystems, Foster City, CA).
Univariate analyses of the genotypes were carried out by fitting a Cox model under the assumption of proportional hazards. A likelihood-ratio test was used to formally test if the estimated hazard ratios were different to the reported values. Statistical analyses were carried out using STATA10, 2009, StataCorp, TX, USA.
Results
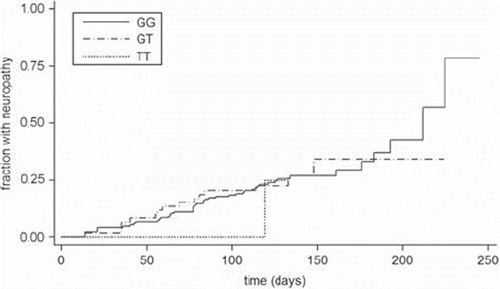
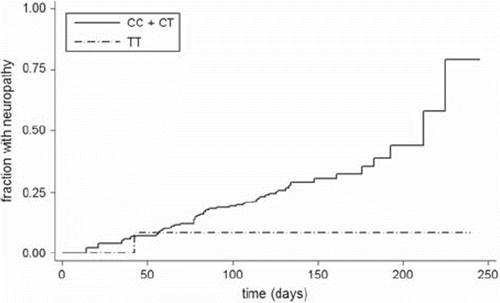
In total 241 patients were included in the study. In four cases the genotype could not be obtained (two for each gene). No departure from Hardy- Weinberg equilibrium was observed. Neuropathy grade 2 or worse was registered for 65 patients (27%). See for neuropathy distribution across genotypes. Neuropathy hazard ratios for RWDD3 (per allele) and TECTA (wt/wt+wt/var vs. var/var) were 0.95 [95% CI 0.57–1.58], p-value 0.84 (see ) and 0.21 [95% CI 0.03–1.53], p-value 0.12 (see ), respectively. For TECTA using the trend model (wt/wt vs. wt/var vs. var/var) the neuropathy hazard ratio was 0.98 [95% CI 0.65–1.46], p-value 0.91. P-values for the likelihood-ratio test of equality between obtained hazard ratios and reported ones were 0.0628 and 0.0007 for RWDD3 and TECTA, respectively.
Table I. Observed frequencies of patients with neuropathy grade 2+ by RWDD3 and TECTA genotype.
Discussion
The results of this replication study clearly indicates that the association between the SNPs rs2296308 in RWDD3 and rs1829 in the intron of TECTA and time-to-neuropathy in paclitaxel treatment reported by Schneider et al. was not found in our population. For RWDD3 the hazard ratio was 0.95 compared to the hazard ratio of 1.5 in the initial report. As the upper limit of the 95% confidence interval in our study was 1.58, and because the p-value for the likelihood-ratio test was only close to significant (0.0628) we cannot decidedly rule out an association of the magnitude reported by Schneider et al., but it seems very unlikely. For TECTA the hazard ratio was 0.21 compared to 2.07 in the initial report. Interestingly, our data suggests a trend towards a protective effect (p-value 0.12) of the var/var genotype compared to other genotypes; i.e. in contrast to the initial report. The likelihood-ratio test was highly significant (p-value 0.0007) suggesting that the true hazard ratio is different from the reported value of 2.07.
Little is known about RWDD3 and TECTA but they have been implied to play a role in neurosensoric hear loss and cellular stress [Citation7] which makes them plausible candidates for neuropathy susceptibility, but more studies, including functional studies are clearly warranted.
Dogmatically, three possible reasons for a failure to replicate should be considered: 1) Type 1 error in our study, i.e. falsely rejecting the hypotheses that the true effects coincide with the reported ones. However, the confidence intervals for the hazard ratios excluded or nearly excluded the originally reported hazard ratios. This suggests that a type 1 error is unlikely. However, due to the modest size of our study population (241 vs. 2204 in the initial report) and because of the unexpected protective effect of TECTA further validation studies are warranted. 2) Overestimation in the original report, i.e. that the observed correlation between SNPs and time to neuropathy was mainly a chance finding. The risk of overestimation is imminent to any GWAS, as no corrections are made. Hence our result may just illustrate the need for validation studies. 3) That the two studied populations differ in ways that invalidates the comparison. Notably, paclitaxel was administered every week for 12 weeks in the primary study as opposed to every third week for 18–27 weeks in our study. Paclitaxel given every third week with higher paclitaxel dose than weekly paclitaxel possible has different neuropathic potential and the two studies also differ with regard to adjuvant chemotherapy. In spite of different regimen the prevalence of grade 2–4 neuropathy was remarkably similar: 27% in this study compared to 28% in the primary study. The primary study found an effect of ethnicity and age on time to neuropathy. Our population was exclusively Caucasian and age was not a significant covariate in our population; hazard ratio 1.02 [95% CI 0.99–1.04], p-value 0.19. For completeness age was included in the Cox model, but changes were insignificant (data not shown).
In conclusion, this study clearly failed to replicate a GWAS reporting an association between the two SNPs rs2296308 in RWDD3 and rs1829 in the intron of TECTA and time to neuropathy in patients treated with paclitaxel. Unexpectedly, the TECTA var/var genotype suggested protection rather than susceptibility to neuropathy, but this finding itself warrants replication.
Acknowledgements
We wish to acknowledge the AGO Ovarian Cancer study group in Wiesbaden, Germany and The Nordic Society of Gynaecologic Oncology, Clinical Trial Unit, Odense, Denmark for making available the clinical data from the AGO-OVAR-9 study and the NSGO-OC9804 (TEC) study.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the Danish Ministry of Interior Affairs and Health (2001–2007) [J.nr 2006-12103-276], the European Commission [CHEMORES LSHC-CT-2007-037665], the Swedish Cancer Society, Swedish Research Council, and the Danish Research Agency [J.nr 271-05-0266].
References
- Karlan B, Markman M, Eifel P. Gynecologic cancers. In: DeVita V, Hellman S, Rosenberg S, editors. Principles and practice of oncology, 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004.
- Beitelshees AL, Veenstra DL. Evolving research and stakeholder perspectives on pharmacogenomics. JAMA 2011;306: 1252–3.
- Schneider B, Li K, Miller K, Flockhart DA, Radovich M, Hancock BA, et al. Genetic associations with taxane-induced neuropathy by a genome-wide association study (GWAS) in E5103. ASCO Meeting Abstracts Suppl abstract 1000[29] 2011.
- Bergmann TK, Brasch-Andersen C, Green H, Mirza MR, Pedersen RS, Nielsen F, et al. Impact of CYP2C8*3 on paclitaxel clearance: A population pharmacokinetic and pharmacogenomic study in 93 patients with ovarian cancer. Pharmacogenomics J 2011;11:113–20. Epub 2010 Apr 6.
- Bergmann TK, Green H, Brasch-Andersen C, Mirza MR, Herrstedt J, Hølund B, et al. Retrospective study of the impact of pharmacogenetic variants on paclitaxel toxicity and survival in patients with ovarian cancer. Eur J Clin Pharmacol 2011;67:693–700.
- Green H, Soderkvist P, Rosenberg P, Mirghani RA, Rymark P, Lundqvist EA, et al. Pharmacogenetic studies of paclitaxel in the treatment of ovarian cancer. Basic Clin Pharmacol Toxicol 2009;104:130–7.
- Online Mendelian Inheritance in Man. 2012. [cited 28 Mar 2012]. Available from: http://www.omim.org/entry/602574.