Abstract
Background. To evaluate long-term local control, survival, radiation side effects and functional outcome after limb-sparing surgery followed by postoperative radiotherapy (RT) for soft tissue sarcoma (STS). Material and methods. Between 1995 and 2010, 118 patients with STS of an extremity were treated with limb-sparing surgery and postoperative RT. Follow-up was complete for all patients. Acute and late radiation related toxicities were scored using CTCAE v4.0. Results. Median follow-up was 93 months. RT dose was 60 Gy in 92.4% of the patients; 5.1% received 66 Gy; 2.5% 50–56 Gy. Actuarial local recurrence rates at five and 10 years were 9% and 12%. Five- and 10-year overall survival rates were 69% and 51%. Acute radiation toxicities occurred in 91% of the patients; 19% were grade 3, 2% grade 4. Late radiation toxicities were reported in 71% of the patients: 50% grade 1, 18% grade 2, and 3% grade 3. Limb and joint function after treatment were good, 19% having mild limitation of motion, 1.5% moderate, and 2.5% severe limitations. Conclusion. Limb-sparing surgery with 60 Gy postoperative radiotherapy for patients with STS provides excellent local control and high survival rates with acceptable toxicity and functional outcomes.
Soft tissue sarcomas (STS) are a heterogeneous group of rare malignancies arising in mesenchymal tissues. The incidence of STS in the Netherlands is 2.8 per 100 000 (ESR) [Citation1]. Most STS (60%) are localized in the extremities [Citation2]. Therapeutic goals in the treatment of STS of the extremities are maximizing tumor control and survival with preservation of optimal limb and joint function [Citation3,Citation4]. For most patients limb-sparing surgery is feasible, and adjuvant radiation therapy is used to improve local control in patients with adverse factors such as marginal excision, high grade, deep localization, recurrent disease at presentation [Citation5]. Several studies showed similar outcomes after limb-sparing surgery with radiation therapy (RT) compared to amputation, both with respect to local control and survival [Citation3,Citation4,Citation6,Citation7]. An analysis of the SEER database [Citation4] reported improved survival of patients with high grade STS if limb-sparing surgery was combined with RT (three-year overall survival 73% vs. 63%, p < 0.001). Previous studies using adjuvant RT reported five-year local control rates after limb- sparing treatment of 82–95%, and five-year survival rates of 70–76% [Citation4–7].
Patient functioning after treatment for STS of the extremities is an important issue, because impairment and functional disabilities cause reduced quality of life [Citation8]. In a review of functional outcomes of limb-sparing treatment, approximately 50% of the patients had functional impairments, although not always resulting in limitations in daily activities [Citation9].
The present analysis was done to evaluate long-term results of limb-sparing surgery and postoperative RT for STS of the extremities with regard to local control and survival. Acute and late radiation-related toxicities were analyzed with regard to functional outcomes.
Material and methods
Patient selection
A total of 338 patients were treated with radiotherapy for a mesenchymal tumor of soft tissue at the Department of Clinical Oncology, Leiden University Medical Center (LUMC, a regional referral center for treatment of STS) between January 1995 and April 2010. Patients were excluded if they had non-extremity STS, if they had been treated with palliative intent, or if they had a histological subtype with a different treatment approach or failure pattern such as desmoid-type fibromatosis or Kaposi sarcoma. Of the remaining 121 patients treated with limb-sparing surgery and radiotherapy, two were excluded for synchronous distant metastases, and one for preoperative RT, leaving 118 patients in the analysis. All 118 patients were treated with curative intent using limb-sparing surgery and postoperative RT; 12 patients (10%) also received chemotherapy. Details of treatment and outcomes were extracted from the surgical, pathological, and radiological reports and patient files.
A tumor was considered primary STS when it was previously untreated, or if only a biopsy had been performed at time of presentation. When curative treatment was indicated for recurrent STS previously treated with surgery alone, it was considered recurrent STS.
The WHO classification (2002) for soft tissue sarcoma was used to classify the different histologies into groups [Citation10]. Tumor size was divided in three groups: ≤5 cm, >5–10 cm or ≥10 cm. The tumor grade was defined as high (grade III) or low/intermediate (grade I and II). Grading was based on degree of differentiation, number of mitoses per 1.734 mm2 and amount of necrosis according to the Féderation Nationale des Centres de Lutte Contre le Cancer (FNCLCC) system. This grading system is not applicable for some histologic subtypes [such as malignant peripheral-nerve sheath tumors (MPNST) and clear cell sarcomas], which are considered high grade STS because of their clinical behavior. The anatomical depth was superficial if the tumor was located above the superficial fascia, and deep if involving the fascia and/or located beneath the fascia. Margin status was classified as involved (microscopically involved surgical margin), marginal (surgical margin in pseudo capsule or reactive zone), wide (intracompartmental en bloc resection) or radical (extracompartmental en bloc entire compartment).
Recurrent disease was histologically confirmed and defined as local recurrence, regional (inguinal or axillary lymph node) recurrence and/or distant metastasis. Recurrences were evaluated with respect to the radiation fields (in-field, out-of-field or at the edge of the field).
Radiation related side effects were scored using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Radiation side effects were divided into acute and late (> 90 days) effects. In 11 cases, information regarding toxicity was missing.
Treatment
All 118 patients were treated with limb-sparing surgery followed by postoperative radiotherapy, after having been presented and discussed at the weekly multidisciplinary Bone and STS Tumor Board. All histopathology specimens were reviewed by a LUMC sarcoma pathologist, except for the first time period (before 2000), during which it was not done for all patients. Primary surgery had been performed at LUMC and/or regional referring hospitals. In case of re-excision, this was done in LUMC. Standard treatment for STS of the extremities was limb-sparing surgery and radiotherapy, provided that limb function could be preserved. There were minor differences in RT indications, techniques and dose schedules over the years. Radiotherapy was indicated when a marginal or microscopically incomplete resection had been performed. Indications for radiotherapy in low grade tumors were based on patient and tumor characteristics such as recurrent disease at presentation, surgical margins and estimated risk of recurrence.
All patients were treated with external beam radiotherapy using 6–10 MV photons and/or 4–18 MeV electrons from a linear accelerator. Computed tomography (CT)-based three-dimensional (3D) conformal treatment planning was used. Whenever feasible with respect to the clinical target volume (CTV), care was taken to avoid including the full circumference of the extremity in the radiation field; in 12 patients (10%) the full circumference was included. The CTV consisted of the tumor bed with 2 cm margin in the axial directions, and 5 cm in the longitudinal directions, and was adapted to anatomic barriers. The planning target volume included the CTV with a 1 cm margin.
For postoperative radiotherapy during the period of this study, a total dose of 60–66 Gy was given in 2.0 Gy fractions, five times per week: 60 Gy for close surgical margins (less than 1 cm tumor-free margin in the fixed specimen) or 66 Gy for microscopically involved margins.
Statistical analysis
Statistical analyses were done using SPSS version 17.0 (SPSS Inc, Chicago, IL), and were based on data frozen on January 1, 2011. Time intervals to local recurrence or metastases were calculated from date of histological diagnosis to date of recurrence, with censoring at date of death or of last follow-up for patients alive and recurrence-free; survival was calculated from date of diagnosis to date of death irrespective of the cause, with censoring at date of last contact for patients alive. Synchronous local or regional recurrence and distant metastases were observed in five patients; these were considered as separate events in the analysis of local and distant recurrence. Actuarial survival rates were calculated using the Kaplan-Meier method and log-rank test. The competing risks method (with death as competing risk) was used for analysis of local recurrence and distant metastasis. The relationship between local recurrence or metastases and survival was determined using the time-dependent Cox regression analysis. A two-sided p-value of < 0.05 was considered statistically significant.
Results
Follow-up ranged from 9 to 192 months, with a median follow-up of 93.4 months for patients alive. Patient and tumor characteristics are shown in .
Table I. Patient characteristics.
In this study, 97% of the patients received a total dose of at least 60 Gy (109 received 60 Gy, 6 received 66 Gy). One patient (epitheloid sarcoma diagnosed in 1995) had received 50 Gy and two patients had received 54 Gy and 56 Gy (one myxoinflammatory fibroblastic sarcoma and one extraskeletal Ewing sarcoma).
All patients completed their radiation treatment without delay. In 73 patients (62%), a joint had been included in the radiation field. In order to preserve range of motion and prevent or treat lymph edema, patients were referred for physiotherapy.
The use of chemotherapy was individualized and mostly based on clinical trials. Twelve patients received chemotherapy: neo-adjuvant (n = 9), adjuvant (n = 2) or both (n = 1). Among the nine patients with neo-adjuvant chemotherapy, eight received isolated limb-perfusion for an initially unresectable tumor.
Patterns of failure
Twelve patients developed local recurrence and seven developed regional recurrence (none had both). The actuarial five- and 10-year local recurrence rates were 9% and 12%, respectively. The interval from completion of postoperative radiotherapy to the development of local recurrence ranged from 6 to 66 months, with a median of 16 months. Characteristics and treatment results of patients with local and regional recurrences have been specified in . All patients with recurrences had received a radiation dose of 60 Gy.
Table II. Characteristics and treatment results of patients with local and regional recurrence.
All patients with local recurrences received salvage treatment and nine (75%) remained recurrence-free during further follow-up. Among the patients with a local recurrence, 67% developed distant metastasis, compared to 86% of the patients with a regional recurrence.
Distant metastases were diagnosed in 40 patients (34%), mostly localized in the lungs (78%). The actuarial five-year and 10-year rates of distant metastasis were 31% and 37%. The interval from postoperative radiotherapy to distant metastasis ranged from a month to more than 10 years, with a median of 12 months and a mean of 22 months. Seven patients with local or regional recurrence had synchronous distant metastases. Most patients with metastases (31/40, 77%) received active treatment. Several treatment modalities (chemotherapy, surgery, radiotherapy, biologicals) were given as single or combination therapies at the time of metastases. Twelve (48%) of 25 patients with isolated lung metastases had thoracic surgery (with or without presurgical chemotherapy). Median survival after diagnosis of isolated lung metastases for these 25 patients was 1.15 years (range 0.1–14.6 years).
Survival
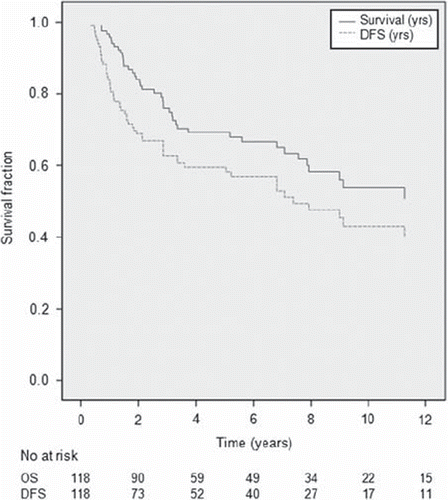
Actuarial overall and disease-free survival rates are shown in . Five- and 10-year overall survival rates were 69% and 51%, and disease-free survival rates were 64% and 44%.
A total of 42 patients had died; 30 deaths were disease-related (27 distant failures, one local progression, two combined). One death was related to chemotherapy for metastatic disease. Nine deaths were due to other causes. Two causes of death were unknown, but without previous relapse.
Among the 76 patients alive, 62 had never had disease recurrence; four were alive after diagnosis of local recurrence, six after distant metastases, and four after both. Patients with a local recurrence had a 2.8 times higher risk of death compared to those without recurrence (CI 1.16–6.7, p = 0.022). Patients with distant metastases had a hazard ratio for death of 16 compared to those without metastases (CI 8.1–31.5, p < 0.001).
Prognostic factors
summarizes the relationship between local control and patient and tumor characteristics. In univariate analyses, tumor site was the only significant prognostic factor, with upper extremity STS having an increased risk of local recurrence compared to lower extremity STS (24% vs. 6% local recurrence, p = 0.04). Tumor size (divided in three groups: ≤5 cm, > 5–10 cm, >10 cm) did not reach significance as prognostic factor (p = 0.16); if size ≤ 5 cm vs. >5 cm was evaluated, there was a trend (p = 0.09) for increased risk of local recurrence.
Table III. Univariate analysis for local recurrence.
In univariate analyses for distant metastases (data not shown), tumor size >5 cm was the only variable significantly (p = 0.018) associated with the risk of metastases. Multivariate analyses could not be performed, as both for local control and distant metastases only one factor reached significance.
shows the relationship between patient and tumor characteristics and survival. Older age (> 50 years) and close or involved resection margins were associated with a trend towards a lower survival rate.
Table IV. Univariate analysis for overall survival (OS).
Radiotherapy related side effects and functional outcomes
Most patients (91%) experienced some acute toxicity during postoperative radiotherapy. Among these, 36% were grade 1, 34% grade 2, 19% grade 3 and 2% grade 4 toxicities (grade 4 were two patients with skin necrosis or wound dehiscence needing a graft). Skin reactions were the most common: 87% rash/dermatitis (67% grade 1–2) and 7.6% wound infections. Eight patients had a wound dehiscence during or just after completion of radiotherapy, five of whom previously had wound healing problems after surgery. None of these patients had received isolated limb-perfusion. Other acute toxicities were grade 1 fatigue (16.9%) and grade 1 limb edema (27%). Six of 32 patients with edema during radiotherapy had edema in the postsurgical phase. Three of 32 (9%) patients with edema had received isolated limb-perfusion.
Late radiation related toxicities are summarized in . Most prominent were late musculoskeletal toxicities. The majority of patients (55%) developed any grade of fibrosis, most were mild (39% grade 1). Twenty-three percent experienced some decrease in joint motion: 18.6% had mild and 1.7% moderate limitations; only 2.5% had grade 3 limitation of joint motion with contracture. Three of 27 patients with decrease in motion had been treated with isolated limb perfusion, these toxicities were grade 1 (n = 2) and grade 2 (n = 1). In 16 (59%) patients with decreased range of motion, a joint had been included in the radiation field. Limb edema occurred in 30 patients (25%); all were grade 1, and 16 also had edema as acute toxicity, and three had received isolated limb perfusion. In five of these 30 patients (17%) with persistent edema, the entire circumference of the extremity had been included in the radiation field.
Table V. Late radiation toxicities according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Discussion
In this analysis of 118 patients with STS of the extremities treated with limb-sparing surgery and postoperative radiotherapy with curative intent, the five-year local control rate was 90%. This compares favorably to reported local control rates, taking into account that 24% of the patients had involved resection margins. In a systematic review [Citation2], local control for STS of the extremities after surgery and adjuvant RT was 90%. However, reported local control rates were lower in case of microscopic positive surgical margins (80%), and for grossly visible tumor of the margins (70%) [Citation5].
In our series, distant metastases (34%) were more frequently diagnosed than local or regional recurrences. Pisters [Citation11] reported a local recurrence rate of 17% and a distant metastatic rate of 22% in a series of 1041 STS patients treated between 1982 and 1994.
The probability of a nodal recurrence as first failure site of STS is low; some histological subtypes (such as clear cell sarcoma) are more likely to metastasize to the regional lymph nodes than others [Citation12]. Riad et al. reported a lymph node recurrence rate in STS of the extremity of 4% [Citation13]. In our study, lymph node recurrence was the first site of recurrence in 6%. We found regional recurrences in two of three patients with clear cell sarcoma.
Overall survival rates were 69% and 51% at five and 10 years, in accordance with reported survival rates [Citation6,Citation14]. This is one of the few studies reporting 10-year survival rates.
Few prognostic factors could be identified in our analysis, probably due to the relatively small sample size. Tumor site was the only significant prognostic factor for local recurrence, with higher recurrence rates for STS of the upper extremity. Alektiar [Citation14] also reported lower local control rates for STS of the upper extremity, probably due to the fact that in the upper extremity it is more difficult to obtain wide surgical margins. Other reported adverse prognostic factors for local recurrence are older age, recurrent disease, involved surgical margins and histological subtypes such as MPNST [Citation11]. We found a trend towards better local control for tumor size ≤5 cm.
Reported adverse prognostic factors for distant metastasis are histological grade, size, deep tumor location and presentation with recurrent disease [Citation11]. In our analysis, tumor size >5 cm was the only factor showing a significantly increased risk of metastases.
Radiation dose has been shown to significantly impact on the risk of local recurrence. Delaney [Citation5] demonstrated in a retrospective study improved local control and survival rates with RT doses >64 Gy in both STS of the extremity and non- extremity sites after surgery with involved margins. Patients who had a wide excision were treated with a slightly lower dose (60 Gy) without compromising local control and with fewer long-term toxicities [Citation15]. At the time, these data were the reason for us to increase the dose from 60 to 66 Gy for involved margins. However, excellent local control was achieved in our series with 60 Gy, with only 4% of the patients having received 66 Gy, while 24% had microscopically involved margins.
The large majority of patients (91%) experienced acute toxicities, and 70% were mild, CTC grade 1 or 2. Reported rates of wound healing problems among patients who had postoperative RT vary from 9% to 17% [Citation16,Citation17], and was 7% in our analysis. Most (five of eight) patients with wound healing problems already had complications in the postsurgical phase.
Essential for functional outcome after treatment are late radiation-related toxicities, because these impact most on daily activities and quality of life. Among the 118 patients, 71% reported any late toxicity, 68% were CTC-grade 1 or 2. Fibrosis was the most common toxicity (55%). Decrease of range of joint motion was found in 23%, however this was mostly mild, with only 2.5% CTC-grade 3 limitations. Edema was reported in 25% of the patients, some of whom also had postsurgical limb edema.
In a randomized trial of pre- vs. postoperative RT reported by Davis [Citation18], postoperative RT to a dose of 66 Gy resulted in more late radiation toxicities, especially subcutaneous fibrosis, joint stiffness and extremity edema, than preoperative RT to a dose of 50 Gy. Using EORTC/RTOG Late Radiation Toxicity Criteria (1995), fibrosis grade 1 was reported in 52% of patients, grade 2 or more in 48%; joint stiffness of at least grade 2 in 23%; and edema grade 2 or greater in 23% of patients treated with postoperative RT. All patients experienced some degree of toxicity and the level of toxicity affected patient functioning. In the current analysis a lower proportion of the patients experienced late toxicities, and these were less severe, with only 15% grade 2 or more fibrosis and 4% grade 2 or more joint stiffness. The radiation dose used in our study was 60 Gy (only six patients receiving 66 Gy), while all patients in Davis´ study had received 66 Gy, which could partly account our lower toxicity rates. However, a limitation of our analysis was the fact that it was a retrospective study, and toxicity data had to be scored from the medical files. Presently, we are using 50 Gy pre-operative RT more often, to limit both total dose and irradiated volume, in order to further reduce the risk of severe fibrosis and limitations in movement.
In conclusion, limb-sparing surgery with postoperative radiotherapy for patients with STS provides excellent local control and high survival rates, with acceptable toxicity and good functional outcome. Although Delaney [Citation5] reported increased local control rates with dose levels > 64 Gy, in our study 60 Gy seemed sufficient for local control (90%), even in cases of (microscopically) involved surgical margins.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dutch Cancer Registry. [cited 2011 Mar 28]. Available from: http://www.ikcnet.nl/cijfers/index.php.
- Strander H, Turesson I, Cavallin-Stahl E. A systematic overview of radiation therapy effects in soft tissue sarcomas. Acta Oncol 2003;42:516–31.
- Muhic A, Hovgaard D, Mork PM, Daugaard SS, Højlund Bech BB, Roed HH, et al. Local control and survival in patients with soft tissue sarcomas treated with limb sparing surgery in combination with interstitial brachytherapy and external radiation. Radiother Oncol 2008;88:382–7.
- Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: A SEER analysis. Int J Radiat Oncol Biol Phys 2010;77:203–9.
- DeLaney TF, Kepka L, Goldberg SI, Hornicek FJ, Gebhardt MC, Yoon SS, et al. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys 2007;67:1460–9.
- Fein DA, Lee WR, Lanciano RM, Corn BW, Herbert SH, Hanlon AL, et al. Management of extremity soft tissue sarcomas with limb-sparing surgery and postoperative irradiation: Do total dose, overall treatment time, and the surgery-radiotherapy interval impact on local control?Int J Radiat Oncol Biol Phys 1995;32:969–76.
- Yang JC, Chang AE, Baker AR, Sindelar WF, Danforth DN, Topalian SL, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998;16: 197–203.
- Schreiber D, Bell RS, Wunder JS, O’Sullivan B, Turcotte R, Masri BA, et al. Evaluating function and health related quality of life in patients treated for extremity soft tissue sarcoma. Qual Life Res 2006;15:1439–46.
- Davis AM. Functional outcome in extremity soft tissue sarcoma. Semin Radiat Oncol 1999;9:360–8.
- Fletcher CDM, Rydholm A, Singer S, Sundaram M, Coindre JM. WHO classification of soft tissue tumours. In: Fletcher CDM, Unni KK, Mertens F, editors. World health organization classification of tumours. Pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press; 2002. p. 9–18.
- Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996; 14:1679–89.
- Zagars GK, Ballo MT, Pisters PW, Pollock RE, Patel SR, Benjamin RS. Surgical margins and reresection in the management of patients with soft tissue sarcoma using conservative surgery and radiation therapy. Cancer 2003;97:2544–53.
- Riad S, Griffin AM, Liberman B, Blackstein ME, Catton CN, Kandel RA, et al. Lymph node metastasis in soft tissue sarcoma in an extremity. Clin Orthop Relat Res 2004;129–34.
- Alektiar KM, Brennan MF, Singer S. Influence of site on the therapeutic ratio of adjuvant radiotherapy in soft-tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 2005; 63:202–8.
- Mundt AJ, Awan A, Sibley GS, Simon M, Rubin SJ, Samuels B, et al. Conservative surgery and adjuvant radiation therapy in the management of adult soft tissue sarcoma of the extremities: Clinical and radiobiological results. Int J Radiat Oncol Biol Phys 1995;32:977–85.
- O’Sullivan B, Davis AM, Turcotte R, Bell R, Catton C, Chabot P, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002;359:2235–41.
- Cannon CP, Ballo MT, Zagars GK, Mirza AN, Lin PP, Lewis VO, et al. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006;107:2455–61.
- Davis AM, O’Sullivan B, Turcotte R, Bell RR, Catton CC, Chabot PP, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005;75: 48–53.