Abstract
Background. There are few reports concerning treatment strategies and their contributions to survival of patients with malignant mesothelioma (MM) in Japan. Material and methods. We extracted all death cases due to MM between 2003 and 2008. The diagnosis of MM was confirmed in 929 cases. Histological subtypes was determined in 709 cases, including 396 (55.9%) epithelioid, 154 (21.7%) sarcomatoid, 126 (17.8%) biphasic, and 33 (4.7%) other types. Results and conclusion. Median overall survival (OS) of all MM cases was 7.7 months (95% confidence interval, 7.1–8.3). Median OS of patients with epithelioid MM was significantly longer than that of patients with biphasic (p = 0.030) or sarcomatoid (p < 0.001) MM. Surgical resection was performed in 172 patients (18.5%) and 449 (48.3%) received systemic chemotherapy. Survival of patients treated with both surgery and systemic chemotherapy was favorable. Median OS of patients in the late phase of the study period (2006–2008) was significantly longer than that in the early phase (2003–2005) (8.1 vs. 7.5 months, p = 0.008). Independent favorable prognostic factors included age younger than 70 years, female gender, epithelioid subtype, and clinical stage I–III. Multivariate analysis demonstrated that patients who had radical surgery and systemic chemotherapy showed a longer survival, though this could be due to selection bias of patients.
Malignant mesothelioma (MM) is an aggressive tumor that develops from mesothelial cells of the pleura, peritoneum, pericardium, or testicular tunica vaginalis. It is generally associated with a history of asbestos exposure [Citation1] and has a very poor prognosis [Citation2]. Management of MM is controversial, and there is no definitive standard of care. Until recently, individual modalities such as surgery, radiotherapy, and chemotherapy have failed to prolong survival. Intensive multi-modality treatment including extrapleural pneumonectomy (EPP), systemic chemotherapy, and radiotherapy has been evaluated at several institutions with encouraging results [Citation3]. However, this approach is feasible only for a small proportion of patients. Reported adverse predictors of survival in patients with MM are sarcomatoid histology, older age, advanced International Mesothelioma Interest Group (IMIG) stage, and absence of surgery or chemotherapy [Citation4]. However, most of this evidence is reported from Europe, and there are few reports concerning the clinical features of MM in Japan. A newspaper article published in June 2005 reported that five residents who lived near the now-closed asbestos cement pipe plant in Amagasaki, Japan, developed pleural mesothelioma. Since this report, asbestos-related problems have raised significant social concern. We performed a nationwide retrospective survey to investigate all MM cases in Japan. As a result, we analyzed more than 6000 MM cases registered in the Vital Statistics yearly survey carried out by the Japanese Ministry of Health, Labour, and Welfare, between 2003 and 2008. To our knowledge, this is the largest study concerning MM in Japan. We have already reported the clinical features of MM [Citation5]. We confirmed that more than 70% of MM cases in Japan were associated with asbestos exposure.
In this paper, we report the treatment and survival data of patients with MM in Japan. The aim of this study was to characterize the treatment strategy and its contributions to survival. Changes in treatment modality and survival data within the study period were examined. Prognostic factors including age, gender, histological subtype, and treatment modality are also analyzed.
Material and methods
Patients
Methods of this retrospective survey were previously described [Citation5]. In brief, we requested and received authorization to view the death records of Vital Statistics in Japan, and extracted all cases of death due to MM between 2003 and 2008. There were 6030 deaths due to MM. We contacted the closest living relatives of each case to obtain consent for our study by postal mail. As a result, the informed consent was obtained by postal mail from 2069 cases (34.3%). Based on authorization from relatives, we contacted each patients’ medical institution to obtain information including medical records, x-ray films, and/or computed tomographic (CT) images by postal mail. Data from 1111 cases were obtained. We reviewed the medical records and radiological images to confirm the clinical and pathological diagnosis of MM. As a result, the clinical diagnosis of MM was confirmed in 929 cases, including 753 males (81.1%) and 176 females (18.9%). Median age at diagnosis of MM was 67.0 years (range, 16–94). The origin of MM was the pleura in 794 cases (85.5%), peritoneum in 123 cases (13.2%), pericardium in seven cases (0.8%), and testicular tunica vaginalis in five cases (0.5%). Histological subtypes of MM based on World Health Organization criteria [Citation6] was determined in 709 cases, included 396 (55.9%) epithelioid, 154 (21.7%) sarcomatoid, 126 (17.8%) biphasic, and 33 (4.7%) other types. The clinical stage of pleural MM was determined according to IMIG criteria [Citation7] in 603 cases, including 172 cases (28.5%) of stage I and II, 279 cases (46.3%) of stage III, and 152 cases (25.2%) of stage IV.
Statistical analysis
Mean values were compared using the t-test. Comparisons between independent groups were performed using χ2-test. Survival time was defined as the period from diagnosis to death. Survival curves were calculated using the Kaplan and Meier method. The log-rank test was used to evaluate differences in survival. The Cox proportional hazard model was employed for multivariate analysis. Statistical calculations were performed using SPSS statistical package version 11.0 (SPSS Inc., Chicago, USA).
Results
Treatment of MM
Treatment modalities in 929 MM cases were reviewed. Among them, radical surgical resection was performed in 172 cases (18.5%) and systemic chemotherapy was delivered in 449 cases (48.3%). In 374 cases (40.3%), only palliative treatment was given. Radical surgical resection was performed in 154 (19.4%) of the 794 cases of pleural MM. Among them, EPP was performed in 103 cases (66.9%). Systemic chemotherapy was delivered in 386 cases (48.6%). Among patients that underwent EPP, systemic chemotherapy was delivered in 80 cases (51.9%) as either neoadjuvant therapy, adjuvant therapy, or at disease relapse.
The chemotherapy regimen of the initial treatment was identified in 432 cases. Among them, platinum-based chemotherapy was delivered in 337 cases (78.0%), non-platinum combination chemotherapy in 48 cases (11.1%), and non-platinum monotherapy in 47 cases (10.9%). Platinum-based regimens included cisplatin/gemcitabine in 134 cases (39.8%), cisplatin/pemetrexed in 74 cases (22.0%), carboplatin/gemcitabine in 54 cases (16.0%), carboplatin/paclitaxel in 15 cases (4.5%), and others. The non-platinum combination regimen delivered most frequently was gemcitabine/vinorelbine (37/48, 77.1%). Gemcitabine was the most frequently used non-platinum monotherapy (27/47, 57.4%).
Next, we examined the changes in treatment modalities over time. The proportion of patients treated with surgical resection was 18.2% (110/605 cases) in the early phase (2003–2005) of the study and 19.1% (62/324 cases) in the late phase (2006–2008). The proportion of patients treated with systemic chemotherapy significantly increased from 42.6% (258/605 cases) in the early phase to 59.0% (191/324 cases) in the late phase (p < 0.001). The proportion of patients receiving palliative treatment alone decreased from 43.5% (263/605 cases) in the early phase to 34.3% (111/324 cases) in the late phase (p = 0.005).
Survival of MM
Among the 929 patients with MM, date of diagnosis was unknown in eight cases; survival analysis was performed on the remaining 921 cases. Median overall survival (OS) was 7.7 months [95% confidence interval (CI), 7.1–8.3]. Median (95% CI) OS of patients with pleural MM (n = 789) was 7.9 (7.3–8.5) months and longer than that of patients with peritoneal MM [4.7 (3.8–5.7); n = 123], but the difference was not statistically significant (p = 0.069). Median (95% CI) OS of epithelioid, biphasic, and sarcomatoid types of MM was 9.4 (7.1–10.7), 7.9 (6.6–9.2), and 4.1 (3.2–5.0) months, respectively. Median OS of epithelioid MM was significantly longer than both biphasic (p = 0.030) and sarcomatoid (p < 0.001) MM, and median OS of biphasic MM was significantly longer than that of sarcomatoid MM (p < 0.001).
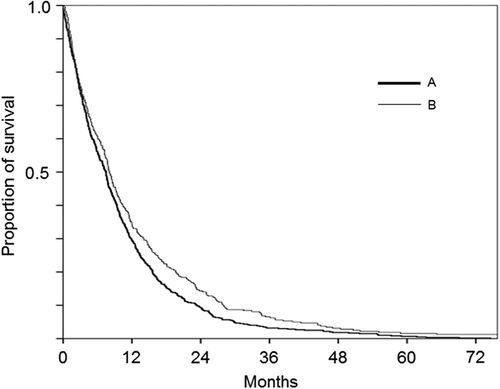
Next, survival was analyzed according to treatment modality. For this purpose, patients were divided into those treated with both radical surgery and systemic chemotherapy (group A), those with radical surgery but no systemic chemotherapy (group B), those with systemic chemotherapy but no radical surgery (group C), and those without radical surgery or systemic chemotherapy (group D). Median (95% CI) OS was 15.1 (12.0–19.0), 8.6 (6.6–10.5), 9.3 (8.4–10.2), and 4.1 (3.4–4.8) months in group A, B, C, and D, respectively. Survival of group A was significantly longer than that of the other groups (A vs. B; p = 0.018, A vs. C and A vs. D; p < 0.001) and the survival of group D was significantly shorter than that of group B (p = 0.008) and group C (p < 0.001).
Survival of pleural MM
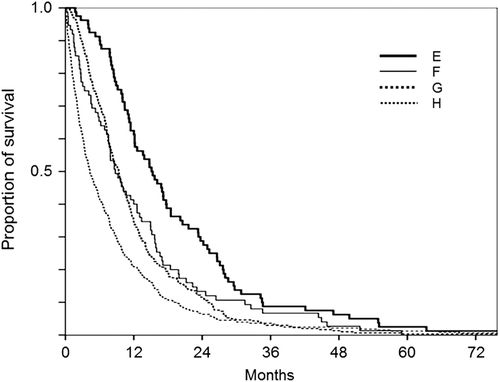
We next analyzed survival of patients with pleural MM only. Histological subtype was determined in 607 cases. Median (95% CI) OS was 10.2 (8.9–11.6), 8.0 (6.2–9.8), and 4.2 (3.0–5.5) months for the epithelioid (n = 325), biphasic (n = 111), and sarcomatoid (n = 141) subtype, respectively. OS was significantly shorter for the sarcomatoid subtype compared to both epithelioid (p < 0.001) and biphasic subtypes (p < 0.001) (). According to IMIG stage, median (95% CI) OS was 11.2 (9.4–13.0), 7.9 (7.1–8.7), and 3.9 (3.0–4.6) months for stages I and II, stage III, and stage IV, respectively. OS of stage III patients was significantly shorter than that of stage I and II (p = 0.001); OS of stage IV patients was significantly shorter than that of stage III (p < 0.001). Patients were then divided according to treatment as those receiving radical surgery and systemic chemotherapy (group E), radical surgery but no systemic chemotherapy (group F), systemic chemotherapy but no radical surgery (group G), and without radical surgery or systemic chemotherapy (group H). Median (95% CI) OS was 15.1 (11.6–18.6), 8.8 (6.7–10.8), 9.4 (8.4–10.4), and 4.3 (3.3–5.3) in group E, F, G, and H, respectively (). Survival of group E was significantly longer than that of the other groups (E vs. F; p = 0.035, E vs. G and E vs. H; p < 0.001) and survival of group H was significantly shorter than that of group F (p = 0.024) and group G (p < 0.001).
Figure 1. Overall survival of patients with malignant pleural mesothelioma according to histological subtype.
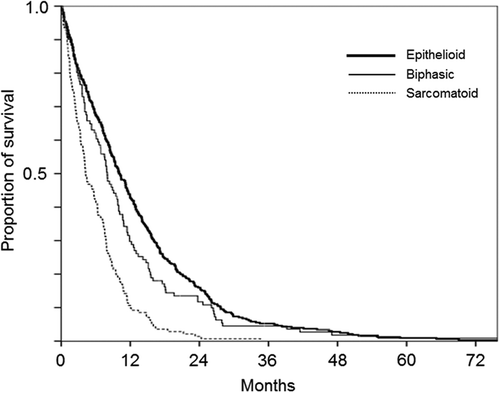
Figure 2. Overall survival of patients with malignant pleural mesothelioma according to treatment modality. E. both radical surgery and systemic chemotherapy, F. radical surgery but no systemic chemotherapy, G. systemic chemotherapy but no radical surgery, and H. without radical surgery or systemic chemotherapy.
Then we analyzed the survival according to the chemotherapy regimen. Median OS of cisplatin/ gemcitabine, carboplatin/gemcitabine, and cisplatin/pemetrexed was 10.2, 10.8, and 7.8 months, respectively. The survival of cisplatin/gemcitabine was statistically superior to that of cisplatin/ pemetrexed (Hazard ratio 1.62, 95% CI 1.06–2.45). The survival of group F was similar to that of cisplatin/gemcitabine (Hazard ratio 0.988, 95% CI 0.97–1.00).
Changes in survival of MM
OS according to year of death was analyzed. Median (95% CI) OS was 6.9 (5.8–8.0), 7.3 (6.1–8.5), 8.3 (6.3–10.3), 8.4 (6.7–10.0), 8.8 (6.8–10.8), and 7.2 (5.9–8.6) months for patients who died in 2003, 2004, 2005, 2006, 2007, and 2008, respectively. OS of patients who died in 2005, 2006, and 2007 was significantly longer than those died in 2003. Median (95% CI) OS of patients who died in the late phase (n = 321) was significantly longer than that of patients who died in the early phase (n = 600) [8.1 months (7.1–9.0) vs. 7.5 months (6.7–8.3), p = 0.008] ().
Prognostic factor analyses
In univariate log-rank analysis age < 70 years, epithelioid subtype, and clinical stage I–III were favorable prognostic factors (). These factors and female gender were independent favorable factors on multivariate Cox regression analysis. Further multivariate analysis was conducted to clarify the contribution of radical surgery and systemic chemotherapy to the survival of pleural MM, these factors were included in the analysis as explanatory variables. Both modalities contributed to longer survival ().
Table I. Univariate analysis of clinical variables and survival of patients with malignant pleural mesothelioma (Log-rank test).
Table II. Multivariate analysis of clinical variables and survival of patients with malignant pleural mesothelioma.
Discussion
We analyzed the treatment and survival data of patients with MM in Japan. The aim of this study was to characterize the treatment modalities, survival, and risk factors affecting OS. There were about 6000 deaths due to MM in our study period. We contacted the closest living relatives of each case to obtain consent for our study by postal mail. And we contacted patients’ medical institution to obtain medical information including medical records, x-ray films, and/or CT images by postal mail. Data from 1111 cases were obtained. We have to accept the low collection rate of postal mail method; however, there is no selection bias through the process of our data collection. We determined the treatment and survival data of 929 MM patients. To our knowledge, this is the largest study conducted in Japan and one of the largest studies of MM worldwide.
We demonstrated that MM carries a poor prognosis with a median OS of only 7.7 months in Japan. Median OS of epithelioid MM was significantly longer than either of biphasic or sarcomatoid MM, and median OS of biphasic MM was significantly longer than that of sarcomatoid MM. We also found that female gender, age under 70 years, epithelioid subtype, and clinical stage I–III were favorable prognostic factors. In previous reports, the Cancer and Leukemia Group B (CALGB) [Citation8] identified pleural involvement, elevated lactate dehydrogenase, poor performance status, chest pain, thrombocytosis, non-epithelial histology, and age older than 75 years as poor prognostic factors. The European Organization for Research and Treatment of Cancer (EORTC) [Citation9,Citation10] reported poor performance status, probable diagnosis of MM, leukocytosis, male gender, and sarcomatoid subtype as indicators of poor prognosis. In the Surveillance, Epidemiology, and End Results (SEER) Program [Citation4], the outcomes for 1475 patients with histologically confirmed MM were analyzed; the most important prognostic factors identified were age, gender, tumor stage, treatment, and geographic area of residence. Our results confirmed that the characteristics of MM in Japan are similar to those in the US and Europe.
Regarding treatment modalities, both radical surgery and systemic chemotherapy contributed to prolonged survival of MM based on multivariate prognostic analysis. These results should be interpreted with caution, as choice of therapy is potentially biased by age, clinical stage, physical condition, and other factors. The role of surgical resection in the management of MM is controversial. Among surgical procedures, EPP, in which the lung and ipsilateral parietal pleura, pericardium, and hemidiaphragm are resected, is considered the only procedure of curative content. It is usually integrated in a multimodality strategy combined with chemotherapy and radiotherapy. In the largest series of the trimodality consisting of EPP, adjuvant chemotherapy, and radiotherapy, median survival was 19 months, and five-year survival was 15%. The best outcome was a median survival of 51 months and five-year survival of 46%, obtained in the subset of the patients with epithelial histology, no extrapleural lymph node metastasis, and negative margins [Citation3]. However, Treasure et al. [Citation11] argued that the primary reason for these positive results was patient selection, and they recently reported a lack of survival advantage with EPP [Citation12]. In our study, patients treated with surgery alone demonstrated almost equivalent survival as those receiving systemic chemotherapy alone. Based on currently available data, we believe that surgery should be applied within an integrated, multimodality strategy combined with chemotherapy and radiotherapy, and physicians should be extremely careful about the indication.
In our analysis, median OS of patients who died in the late phase of the study (2006–2008) was significantly longer than that of patients who died in the early phase (2003–2005). We have two explanations for this survival prolongation. One is increased prevalence of definitive diagnostic confirmation of MM. As we described in a previous report [Citation5], there were many more cases in the early phase in which MM was diagnosed clinically based on radiologic or laboratory findings, without pathological confirmation. For most of these cases, only palliative treatment was delivered even for patients with good performance status (PS). The number of these ‘suspected-MM’ cases decreased in the late phase, possibly due to increased use of accurate pathological techniques such as immunohistochemical analysis [Citation13] and the widespread dissemination of less-invasive diagnostic procedures such as thoracoscopy [Citation14] or laparoscopy. The increase in definitive diagnosis of MM would result in an increased number of cases diagnosed at an earlier stage and in patients with good PS. As a result, the proportion of the patients treated with palliative management alone decreased from 43.5% in the early phase to 34.3% in the late phase. These changes might contribute to the prolongation of survival seen here. Another possibility is the impact of systemic chemotherapy. Platinum-based regimens consisting of cisplatin/gemcitabine or carboplatin/gemcitabine was mainly delivered with limited efficacy [Citation15] before the approval of the antifolate agent pemetrexed in 2007 in Japan. Since then, combination therapy with platinum and pemetrexed is considered a standard regimen based on favorable clinical trial results [Citation16,Citation17]. At the same time, the proportion of cases treated with systemic chemotherapy significantly increased from 42.6% in the early phase to 59.0% in the late phase. The introduction of pemetrexed might explain the increased application of chemotherapy, and contribute to the longer survival in the later phase of the study. In our analysis, the OS of cisplatin/pemetrexed was inferior to that of cisplatin/gemcitabine. This is because our analysis included patients who had died from 2003 to 2008 and pemetrexed was approved in 2007 in Japan, hence the long survivors of cisplatin/ pemetrexed were not included.
In conclusion, we report treatment and survival data of patients with MM in Japan. The survival rate has increased due to improved definitive diagnosis and more widespread treatment. Novel approaches for the early diagnosis and improved treatment strategies, based on the biology of the disease, are essential to further improve survival.
Acknowledgements
This research is mainly due to the research foundation from the Ministry of Health, Labour and Welfare of Japan, 200500129A, 200635021A, 200733015A, 200733015B, 200836010A, 200938007A, and 201032004B. It is a part of the research and development and dissemination projects related to the 13 fields of occupational injuries and illnesses of the Japan Labour, Health and Welfare Organization. The sponsors had no involvement in the study design, collection, analysis and interpretation of the data, writing of the manuscript, or decision to submit the manuscript for publication. The authors would like to thank the living relatives of mesothelioma patients who gave consent to our research, the medical institutions that provided the medical information for the mesothelioma cases and Mrs. Rie Sugimoto, Mrs. Keiko Fujimura, and Miss Naomi Ogura for collecting data.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;17:260–71.
- Muers MF, Stephens RJ, Fisher P, Darlison L, Higgs CM, Lowry E, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): A multicentre randomised trial. Lancet 2008;371:1685–94.
- Sugarbaker DJ, Flores RM, Jaklitsch MT, Richards WG, Strauss GM, Corson JM, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54–63, Discussion 63–5.
- Spirtas R, Connelly RR, Tucker MA. Survival patterns for malignant mesothelioma: The SEER experience. Int J Cancer 1988;41:525–30.
- Gemba K, Fujimoto N, Kato K, Aoe K, Takeshima Y, Inai K, et al. National survey of malignant mesothelioma and asbestos exposure in Japan. Cancer Sci 2012;103:483–90.
- Churg A, Inai K, Samet J. Tumours of the pleura. Lyon: IARC Press; 2004.
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995; 108:1122–8.
- Herndon JE, Green MR, Chahinian AP, Corson JM, Suzuki Y, Vogelzang NJ. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest 1998;113:723–31.
- Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE, Giaccone G. Prognostic factors in patients with pleural mesothelioma: The European Organization for Research and Treatment of Cancer experience. J Clin Oncol 1998;16:145–52.
- Fennell DA, Parmar A, Shamash J, Evans MT, Sheaff MT, Sylvester R, et al. Statistical validation of the EORTC prognostic model for malignant pleural mesothelioma based on three consecutive phase II trials. J Clin Oncol 2005;23: 184–9.
- Treasure T, Tan C, Lang-Lazdunski L, Waller D. The MARS trial: Mesothelioma and radical surgery. Interact Cardiovasc Thorac Surg 2006;5:58–9.
- Treasure T, Lang-Lazdunski L, Waller D, Bliss JM, Tan C, Entwisle J, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: Clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763–72.
- Kushitani K, Takeshima Y, Amatya VJ, Furonaka O, Sakatani A, Inai K. Differential diagnosis of sarcomatoid mesothelioma from true sarcoma and sarcomatoid carcinoma using immunohistochemistry. Pathol Int 2008;58:75–83.
- Walters J, Maskell NA. Biopsy techniques for the diagnosis of mesothelioma. Recent Results Cancer Res 2011;189: 45–55.
- Kalmadi SR, Rankin C, Kraut MJ, Jacobs AD, Petrylak DP, Adelstein DJ, et al. Gemcitabine and cisplatin in unresectable malignant mesothelioma of the pleura: A phase II study of the Southwest Oncology Group (SWOG 9810). Lung Cancer 2008;60:259–63.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636–44.
- Nakagawa K, Yamazaki K, Kunitoh H, Hida T, Gemba K, Shinkai T, et al. Efficacy and safety of pemetrexed in combination with cisplatin for malignant pleural mesothelioma: A phase I/II study in Japanese patients. Jpn J Clin Oncol 2008;38:339–46.