Abstract
Introduction. Over-treatment of low-risk early breast cancer patients with adjuvant systemic therapies is an important clinical challenge. Better techniques are required which can be used to distinguish between the large group of patients with no residual disease after surgery and consequently no benefit of adjuvant treatment, from the smaller group with high relapse risk. A better integration of available prognostic factors might contribute to improved prediction of clinical outcome. Material and methods. The current study included 346 unselected pT1pN0 patients who did not receive adjuvant systemic treatment. In Norway, no patients with this stage were recommended systemic treatment at the time of the study (1995–1998). Histological type, tumour size, grade, vascular invasion (VI), hormone receptor (HR) status, HER2 and Ki67 (cut-off 10%) were analysed. Median follow-up was 86 months for relapse and 101 months for death. Results. Thirty-eight patients experienced relapse, 31 with distant metastasis. Twenty-one patients died of breast cancer. In univariate analysis grade, HER2, HR, VI and Ki67 had impact on clinical outcome (p < 0.005, log rank). In multivariate analysis, only grade 1–2 vs. grade 3, HER2, VI, and Ki67 status were significant for disease free survival, distant disease free survival, and/or breast cancer specific survival. These factors were used in combination, to separate patients into groups based on the number of unfavourable factors present [combined prognostic score (CPS) 0–4]. Close to 2/3 of the patients (61.4%) had no unfavourable factor (CPS0), whilst 18.4% had CPS ≥ 2. Only 3.6% of those with CPS0 developed metastasis (p < 0.001). The outcome was clearly worse for patients with CPS ≥ 2 (p < 0.001), systemic relapse was detected in approximately 40%. Conclusions. This study indicates that the combined use of grade, VI, HER2 and Ki67 identifies a subgroup of breast cancer patients with a relapse risk that may question the benefit of adjuvant systemic therapy.
Early detection and increased use of adjuvant systemic treatment have substantially contributed to improved survival in breast cancer [Citation1]. Most breast cancer patients have no evidence of axillary node metastases and overall the prognosis at this stage is good. However, several primary tumour characteristics affect the risk of having minimal residual disease after primary surgery and therefore the probability for future distant metastases. Furthermore, the tumour size itself, reflecting the time dependency of tumour dissemination, may also contribute to the risk of future relapse [Citation2,Citation3]. The availability of prognostic and predictive factors, as well as adjuvant systemic therapies with well documented effects in both node positive and node negative breast cancer, have contributed to the more extended use of adjuvant treatment. Most guidelines have recommended systemic treatment for node positive disease and/or tumour sizes greater than 1–2 cm, irrespective of other tumour characteristics [Citation4,Citation5]. In addition, histological grade, hormone receptor status, HER2 status and vascular invasion have been included as basis for adjuvant treatment decisions. For estrogen receptor (ER) positive patients, use of hormonal treatment for all tumour sizes has also been proposed [Citation6,Citation7].
Based on the current Norwegian guidelines, use of adjuvant systemic treatment includes patients with 6–8% risk of future metastasis [Citation8]. A large number of node negative patients can be grouped into such a risk category, based on, e.g. the presence of a pT1c tumour of grade 2, without further consideration of the tumour biology [Citation9]. Increased screening awareness has also contributed to earlier detection of breast cancer, which has resulted in smaller tumours being identified. This in itself may further increase the number of low-risk patients receiving systemic treatment to the point were there is more than a 90% probability of being over-treated.
In recent years, several gene signatures have shown promising results both as prognostic and as predictive factors (i.e. Mammaprint, Genomic grade, Rotterdam signature, Oncotype Dx, Intrinsic subtypes/PAM50) [Citation10–16]. Some of these are prospectively tested as treatment decision tools in ongoing clinical trials. It has been shown that common prognostic information from various gene signatures is linked to up-regulation of genes related to proliferation [Citation17]. For routine histology specimens, analysis of Ki67 expression can be used to asses the proliferative status of the primary tumour. At the St Gallen Consensus Conference in 2009, the Ki67 labelling index was included as an important factor when deciding on whether to use chemotherapy, in addition to endocrine therapy, in hormone-receptor-positive breast cancers [Citation6].
Further exploration of how combined use of presently available prognostic factors on routine specimens is required. This exploration may identify the large group of patients who have an excellent outcome but with only questionable benefit of adjuvant treatment. This identification is also important for future comparison to the clinical value of gene signatures, which are not yet broadly available due to cost and/or approval issues.
In the prospective observational Oslo1 Micrometastasis Project, no patients with pT1pN0 tumours received adjuvant systemic treatment. This study investigated the clinical significance of several prognostic factors including HER2, vascular invasion and Ki67 analysis on primary tumour formalin fixed paraffin embedded material.
Material and methods
Patients
Patients with pT1pN0 status included in the Oslo Micrometastasis Project were included in the current analysis (n = 346). According to the prevailing guidelines at the time of inclusion to the study, no subgroup with pT1pN0 status received adjuvant treatment. The Oslo Micrometastasis Project enrolled a total of 920 patients in the period from 1995 to 1998, including patients with localised operable breast cancer. Studies of the prognostic significance of disseminated tumour cells (DTC) have been reported previously [Citation18]. Systemic treatment followed the Norwegian Guidelines 1995–1998 and was consequently given to the pT2pN0G2–3 or pN + patients. Chemotherapy (CMF regimen) was administered if age < 55 years or if age 55–65 years and negative hormone receptor (HR) status. HR positive patients received tamoxifen for five years [Citation19]. Mastectomy was performed in 50.6% of the patients and breast conserving surgery in 49.4%. Whole breast irradiation was administered to 98.4% of those receiving breast conserving surgery, and radiotherapy to the chest wall was administered to 3.6% of the mastectomised patients. Relevant clinicopathologic data were extracted from the database of the Oslo Micrometastasis Study Project. The median follow-up time was 86 months (range 5–121 months) for relapse. From the Norwegian Death Cause Registry, additional information was available regarding extended FU time for death. The median FU time for death was 101 months (range 7–127). The study was approved by the Regional Ethical Committee. Written consent was obtained from all patients.
Primary tumour analysis
Primary tumour analyses were performed as earlier described [Citation18]. Histological tumour type, tumour size, grade, and nodal involvement were analysed and classified according to the tumour-node-metastasis (TNM) system (WHO). Grading was centrally performed on whole sections (by EB) according to the recommendations of Elston and Ellis [Citation20]. In cases of doubt, another pathologist (JMN) was consulted. Generally one block was examined per case. The manuscript includes the essential elements of the REMARK criteria [Citation21].
Vascular tumour cell infiltration.
Vascular tumour cell infiltration was assessed on haematoxylin-eosin-stained whole sections from the primary tumours. Blood/lymph vessels were identified morphologically, carefully differentiating them from breast ducts/retraction artifacts. Tumour cells (TC) within vessels mostly formed clusters of various sizes, but ≥ 1 single TC within a vessel was scored as VI, if presence of conclusive TC morphology.
Tissue microarray.
Tissue microarrays were constructed from formalin-fixed paraffin-embedded archival blocks. A pathologist reviewed the haematoxylin-eosin stained slides from these blocks, in order to identify areas of invasive breast cancer and circled out two representative areas from each slide (if possible one from the tumour periphery and one towards the central part). Based on these, two 0.6 mm cores per paraffin block were extracted by a Beecher Instruments Micro Tissue Arrayer. If representative tumour tissue was not present in the initial microarrays, immunostaining of supplementary cores from additional microarrays was performed for the Ki67 analyses.
Immunohistochemistry.
Ki67 Assessment: The tissue microarray (TMA) blocks were sectioned in standard 4 μm paraffin sections. After deparaffinisation in xylene, antigen retrieval was performed in Tris/EDTA buffer pH 9.0 in a microwave oven (Whirpool JT356, 750 watt, 15 min at maximum temperature). Immunostaining was performed by Dako Autostainer Plus, using anti-Ki67 mAb (clone MIB1, product number M7240, Dako; dilution 1:150, incubation time 30 min) and detection system by Dako Envision + System-HRP(DAB). Immunostaining of supplementary cores, when needed, was performed with the same primary antibody; diluted 1:200, pretreated in Dako's PT link pretreatment module, in Tris/EDTA buffer, pH 9.0 for 20 min at 96°C, and detected by Dako's Envision TMFlex/HRP [new immunohistochemistry (IHC) protocol/adjusted to same sensitivity/specificity as initial protocol]. The IHC protocols were adjusted to obtain strong and specific staining on germinal centre cells in tonsillar tissue according to NordiQC guidelines (www.nordiqc.org/Epitopes/ki67/ki67.htm). Ki67 was scored visually by light microscopy. For each tumour, all cells present in the two core sections were evaluated and the average percentage of positive tumour nuclei was calculated by MS and/or EB. In most cases, no major discordances in the Ki67 score between the two cores were observed. Samples containing > 10% Ki67 positive tumour cell nuclei were scored as Ki67-positive, those with ≤ 10% as Ki67-negative, in accordance with other studies [Citation22–23,Citation43]. Of the total of 346 T1N0 non-adjuvant patients, 252 cases had available/ interpretable Ki67 analysis. Of those without Ki67 results, approximately 2/3 (63 of 94) had too little tumour tissue available for extraction. The remaining (31) were excluded from the analysis due to tumours not adequately represented in the TMA cores /sections (< 40 tumour cells or of poor quality).
ER, PR and HER2 Analysis: Immunostaining for estrogen and progesterone receptors (ER and PR) and HER2 was performed as described previously [Citation18]. The initial HER2 immunohistochemical analysis, on whole paraffin sections, was performed prior to today's standardised procedure, using the monoclonal mouse IgG1 antibody CB11 (Biogenex San Ramon, CA, USA) and detected by Ventana ES staining system and Ventana DAB detection kit without antigen retrieval [Citation18]. On the TMA blocks, HER2 FISH analysis was performed according to current standards using HER-2 DNA Probe kit (Vysis Inc., Downers Grove, IL60515, USA) and an Olympus BX61 Fluorescence microscope. Pretreatment of the sections was performed according to an inhouse protocol adapted from Chin et al. 2003 [Citation24]. The primary tumour was regarded positive for ER or PR if ≥ 10% of the TC nuclei were immunostained with the respective antibodies (according to the prevailing recommendations when the study was conducted). The tumour was scored HER2-positive if 10% or more of the TC showed membranous staining and/or if HER2 FISH was positive (HER2/centromer 17 signal ratio ≥ 2.2) [Citation25]. FISH was performed on all tissue available for this purpose.
Statistical analysis
The SPSS software (version 17/18) was used for all statistical analyses. Survival time was measured from the time of surgery to the time of death or first evidence of recurrence. Breast cancer specific survival (BCSS) was measured from the date of surgery to breast cancer-related death, or otherwise censored at the time of the last follow-up, or at the time of a non-cancer-related death. In the same way, disease free survival (time to locoregional systemic relapse) (DFS) and distant disease free survival (time to systemic relapse) (DDFS) were measured. Metastases to the skeleton, liver, lungs, or central nervous system were recorded as systemic relapse. For univariate analyses, Kaplan-Meier survival curves were plotted and p-values were computed by the log-rank test. Cox proportional hazard regression was used for multivariate (stepwise backward elimination) analyses of prognostic effect of relevant variables. All p-values are two-tailed and p < 0.05 considered significant.
Results
Clinicopathological characteristics of the patients
The clinicopathological features of the patients included in the study (n = 346) are presented in . The median age at diagnosis was 58.7 years (range 31.2–87.2 years). Most of these patients had pT1b and pT1c tumours, and the majority were grade 1 or 2 tumours, (38.7% and 44.1%, respectively). Infiltrating ductal carcinoma constituted 75.1% of the cases, while 17.1% were lobular carcinomas. Among the patients, 82.6% were hormone receptor positive (ER and/or PgR positive) and 10.4% were HER2 positive. Tumour cells in vessels were found in 8.7% of the patients. Ki67 IHC expression of > 10% was found in 23.8% of the patients.
. Characteristics of the pT1pN0 patients.a
Univariate and multivariate survival analyses according to histopathological parameters
After a median follow-up (FU) for relapse of 86 months (range 5–121), 38 patients experienced locoregional (n = 7) or systemic (n = 31) relapse. Twenty-one patients died of breast cancer after a median FU time of 101 months (range 7–127). The different clinicopathological parameters presented in were analysed for their prognostic impact. In the univariate analysis, the following factors had a significant impact on outcome: Histological grade, HER2 status, hormone receptor status, vascular invasion and Ki67 (p < 0.001). Menopausal status (based on age, ≥ 55 or < 55 years), histological subtype or pT1 subcategorisation (pT1a, pT1b, pTc) did not affect outcome (, figure legend). Kaplan- Meier survival analyses for disease free, distant disease free and breast cancer specific survival (DFS, DDFS and BCSS, respectively) are presented in . These results show that presence of grade 3, hormone receptor negativity, HER2 positivity, presence of vascular invasion or Ki67 > 10% all individually identified patients with at least a 20% risk of metastasis.
Figure 1. Survival analyses according to primary tumour factors. Kaplan-Meier plots of disease-free survival (DFS), distant disease-free survival (DDFS) and breast cancer specific survival (BCSS) for pT1pN0 patients receiving no adjuvant systemic treatment, according to histological grade, hormone receptor, vascular invasion, HER2 and Ki67 status. All patients with a reported result for the individual marker (see ) are included in the respective survival analysis. In addition pT1 subcategorisation (pT1a, pT1b, pTc), menopausal status, histological subtypes and age (< 55 y vs. ≥ 55 y) were analysed with the following log rank results: pT1a vs. pT1b vs. pT1c: p = 0.134 (DFS), p = 0.224 (DDFS), p = 0.885 (BCSS). Menopause: p = 0.075 (DFS), p = 0.104 (DFSS), p = 0.794 (BCSS). Histological subtypes: p = 0.183 (DFS), p = 0.226 (DDFS), p = 0.421 (BCSS). Age (< 55 y vs. ≥ 55 y): p = 0.325 (DFS), p = 0.340 (DDFS), p = 0.653 (BCSS).
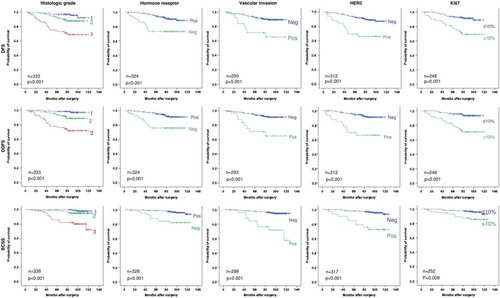
The significant factors in the univariate analysis were included in a multivariate Cox regression analysis. The results are presented in . HER2 and vascular invasion remained significant prognostic factors for all clinical end points, whereas Ki67 was significant for DFS and DDFS, and histological grade for BCSS.
Table II. Cox proportional univariate and multivariate analyses of primary tumour factors for the pT1pN0 patients.
Combined analysis of prognostic factors
Based on the multivariate results, the factors with independent prognostic significance were combined into a “combined prognostic score” (CPS), based on the number of unfavourable factors present. Patients with no unfavourable factor were scored as 0, those with one, two, three or four unfavourable factors, were scored as 1, 2, 3, and 4, respectively. In , the distribution of patients and the respective clinical outcome, according to their CPS, is presented. A large proportion (61.4%) of the patients had no unfavourable factors present (CPS 0), whereas only 18.4% of the patients had CPS ≥ 2. In , Kaplan-Meier survival analyses are presented according to the CPS. Since only two patients had four unfavourable factors, these two patients were merged with those with three unfavourable factors into a combined 3/4 score group. For all end points, the outcome was detrimental for patients grouped into CPS 2, 3 or 4 (p < 0.001), of which approximately 40% developed metastases. In contrast, patients with no unfavourable factor present had an excellent outcome (p < 0.001). Only 3.6% metastases were observed in this group (). Although not statistically significant, those with one unfavourable factor showed a higher number of relapses and breast cancer death than those with none (for DFS p = 0.071). Subgroup analysis of the pT1cpN0 group (n = 147) was also performed. The relapse frequency for patients with CPS 0 was in the same range as for all patients ().
Figure 2. Survival analyses according to combined use of primary tumour prognostic factors. Kaplan-Meier plots of disease-free survival (DFS), distant disease-free survival (DDFS) and breast cancer specific survival (BCSS) for pT1pN0 patients receiving no adjuvant systemic treatment, according to “Combined prognostic score” categorisation. Patients with no unfavourable factor, were scored as 0. Those with one and two unfavourable factors, were scored as 1 and 2, respectively, whereas patients with 3 or 4 unfavourable factors were combined into score 3. Hazard ratios for CPS 0 vs. CPS 1–4 and CPS 0–1 vs. CPS 2–4, respectively, were as follows: DFS: 5.8 (CI 2.5–13.7) and 6.1 (CI 3.0–12.8); DDFS: 7.3 (CI: 2.7–19.5) and 8.9 (CI 3.9–20.1); BCSS: 12.0 (CI 2.7–53.0) and 14.0 (CI 4.5–43.4).
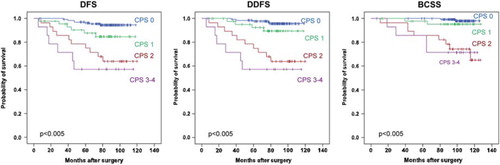
Table III. Clinical outcome in relation to number of unfavourable factorsa detected in pT1pN0 patients (n = 228).
Table IV. Clinical outcome in relation to number of unfavourable factorsa detected in pT1cpN0 patients (n = 147).
The time dependency for the combined prognostic score was tested by Schoenfeld's global test. The test revealed no statistically significant deviation from the proportionality assumption in the Cox proportional hazards model for DFS (p = 0.26), DDFS (p = 0.21) or BCSS (p = 0.26).
Of the pT1pN0 patients with CPS 0, 93% were HR positive. This was significantly higher than the HR positivity among the patients with CPS 1–4 (p < 0.001, χ2). The frequency of metastasis was similar for the HR positive patients with CPS 0 (3.9%) as for all patients with CPS 0, and the Kaplan-Meier survival analyses for HR positive patients according to CPS score did not change from what presented in (data not shown).
Discussion
We analysed an unselected prospectively collected cohort of early breast cancer patients not receiving systemic adjuvant treatment. The frequency of relapse was approximately 10%, showing the overall favourable prognosis for the patients studied. Despite this, today's recommendations for adjuvant systemic treatment would result in treatment of the majority of these patients [Citation4,Citation7]. The present study shows that several primary tumour factors, when used separately, distinguished a larger good prognosis group from a smaller group of patients with relatively poor survival (). However, within these favourable prognostic groups, a significant number of patients still experienced relapse. Our multivariate analysis () showed that grade, vascular invasion, HER2 status and Ki67 expression gave independent prognostic information. We were able to identify a large group of pT1pN0 patients (61%) with no unfavourable prognostic factors present (CPS 0), having a risk of metastasis below 4% and a risk of death close to 1%. For the patients with one unfavourable factor, the prognosis was still very good, but about 9% developed metastasis. Furthermore, among the 70 pT1cpN0G2 patients included in this study, who would all have received adjuvant systemic treatment today, two thirds of the patients were classified as CPS 0 and only 7.1% had CPS ≥ 2 (data not shown). Patients with two or more unfavourable factors had a risk of relapse close to 40%, which clearly emphasises the need of systemic adjuvant treatment. In addition to endocrine treatment for ER positive, in most cases this would include chemotherapy, as both HER2+, high Ki67 and grade 3 indicate potential benefit of chemotherapy [Citation4,Citation7].
Over-treatment affects a large number of patients and raises concerns regarding the toxicity of the adjuvant treatment and the risk/benefit ratio. The benefit of adjuvant systemic chemotherapy for patients with grade 1–2 tumours without vascular invasion, with no HER2 overexpression/amplification and with low Ki67 expression, is therefore uncertain. It can also be questioned whether endocrine adjuvant treatment should be generally recommended for ER positive patients within this group. Of the patients with pT1pN0 CPS 0 status, about 1–2% of the patients would potentially benefit from these treatments, but the side effects affect the majority of the patients, although to a variable degree. This includes cognitive dysfunction, musculoskeletal symptoms, working capabilities, sexual dysfunctions, fatigue, venous thromboembolism (tamoxifen), endometrial carcinoma (tamoxifen), and cardiovascular dysfunction/disease (chemotherapy) [Citation26,Citation27]. For the most commonly used chemotherapy regimen, containing antracyclines, increased cardiac mortality (0.6%), systolic dysfunction (about 5%), and congestive heart disease (10% in elderly), has been reported [Citation26,Citation28]. However, the EBCTCG overview analysis did not find over-mortality for patients receiving anthracyclines [Citation1]. For tamoxifen-treated patients a 2.6% increase in endometrial cancer among postmenopausal patients has been reported, although few deaths were observed [Citation29]. In addition to the undesirable toxicities and economic disadvantages for the individual patient, the cost for the society includes an increased number of people with disabilities, increased social security expenses, and an unsatisfactory use of health care resources, both in terms of medication and personnel. It is critical to reduce over-treatment as much as possible, so that available resources are allocated to patients for whom a reasonable improvement in clinical outcome can be obtained. We are aware of the possibility for late relapses especially among the ER positive patients [Citation1,Citation30]. A longer follow-up time could increase the observed relapse frequency within the study. However, so far we have not observed any systemic relapses beyond the first 6.5 years for patients with CPS 0/1. This indicates that the use of a combination of HER2, Ki67, vascular invasion and grade, can be helpful in the selection of patients who are candidates for restricted or non-use of systemic therapies.
A large number of studies have addressed the prognostic impact of primary tumour factors by IHC/routine histopathology. Several of these have shown that a combination of factors improve the prognostic information [Citation31–34]. However, for untreated patients less data have been published. In a large Danish breast cancer population-based cohort, Christiansen et al. reported survival for patients with pT1pN0G1status not receiving adjuvant systemic treatment [Citation35]. For patients ≥ 60 years of age and tumour size ≤ 10 mm the survival was not different from the Danish general female population, whereas for younger patients and larger tumour sizes a reduction in survival was observed. The study did not include analysis of HER2, Ki67 and VI. Analysis of these factors in addition to grade, could have been able to both “purify” and increase the group of very low-risk patients, by better characterisation of both grade 1 (remove higher risk patients from the group) and grade 2 patients (increase the group by identification of real low-risk grade 2 patients). In a very recent study of 219 premenopausal node negative patients where only 13% received adjuvant systemic treatment, Strand et al. identified a very low-risk group of patients, based on cyclin A as a proliferation marker, in combination with grade, HER2 and ER. Our results are in line with their observations, although the primary tumour markers studied only partially overlap [Citation36].
Ki67 is widely used as a cell proliferation marker in breast cancer studies [Citation37–42], and two recent meta-analyses have reported a statistically significant association between high Ki67 expression and increased risk of breast cancer relapse and death [Citation38,Citation43]. Many of these studies however, use different cut-off points between high and low Ki67 expression [Citation22,Citation43]. In order to improve standardisation, recommendations for Ki67 assessment have recently been published [Citation22]. Our Ki67 analysis has been performed in accordance with these recommendations. We chose to set the cut-off at 10% Ki67-positive cells without knowing how this affected outcome, as the focus was to identify very low-risk patients. This is also in line with other studies [Citation22,Citation23,Citation43]. The Ki67 cut-off set by the St. Gallen recommendation (< 14% vs. ≥ 14%) is based on the Ki67 level that Cheang et al. found to differentiate between Luminal A and Luminal B breast cancer subtypes [Citation7,Citation37]. To separate the luminal subtypes was not the focus of the current study. Furthermore, due to tumour heterogeneity, results from TMA analysis may not be identical to the results from whole paraffin sections. To overcome this problem, inclusion of multiple cores has been recommended. Several investigators have compared Ki67 score on whole paraffin sections and TMA slides, finding comparable results [Citation40,Citation44].
Vascular invasion (VI) of tumour cells is a crucial step in the process of tumour dissemination. Independent prognostic significance of VI has been reported both for lymph node positive and lymph node negative breast cancer patients, and for different histological types including basal and triple negative carcinomas [Citation45–48]. The use of VI was included in the risk categorisation in the St. Gallen recommendations from 2005 [Citation49]. The repeatedly reported significance of VI in various studies, despite large variability in assessment procedures, indicates a robustness of VI as a prognostic factor. However, the methodology for detection of VI needs to be further standardised. In our study we identified blood/lymph vessels morphologically on HE sections, without the use of immunohistochemistry, and obtained, for VI status, the highest hazard ratio in the multivariate analysis (for BCSS; ). Recent studies indicate a benefit of the use of immunohistochemistry to identify VI [Citation46,Citation47] compared to VI analysis performed only on routine hematoxylin/eosin sections. One detected VI focus is reported to be sufficient to affect the prognosis [Citation46]. Further important issues as the number of sections or paraffin blocks to include in the analysis are being investigated [Citation46]. Although the value of VI as a prognostic factor is well supported, there is uncertainty concerning the use of VI as a marker to guide the choice between different adjuvant therapy modalities. Our study indicates, however, that the absence of VI importantly contributes to the identification of patients with a questionable benefit of adjuvant therapy.
Histological tumour grading [Citation20] is standardised and clinically validated. However, the interobserver reproducibility of histological grading is variable among studies [Citation50]. The reproducibility is highest for grade 1 and 3 tumours, but borderline cases of grade 2 represent a challenge, reflecting a biological spectrum of prognostically favourable to more aggressive tumours [Citation50]. This supports the use of additional markers for improved prognostic classification. Analysis of genomic grade index (GGI) may redefine the histological grade 2 tumours into a low-risk (GG1) and a high-risk (GG3) group [Citation10,Citation51]. Further studies incorporating genomic analyses are awaited with interest. The markers used in the current study, may be an indirect way of performing such a reclassification, as the more aggressive genomic grade 3 tumours would predominantly comprise tumours with high Ki67, presence of vascular invasion and/or positivity for HER2.
Gene expression profiling by microarray (i.e. Rotterdam, Mammaprint, Genomic Grade, combined prognostic index) or multi-gene assays (Oncotype Dx) have shown to give important prognostic information in early breast cancer [Citation10,Citation11,Citation13,Citation14,Citation52]. It seems that the overlapping genes/pathways up-regulated in these profiles were related to proliferation [Citation10,Citation17]. Most of these analyses are expensive and need fresh tumour material, which may restrict the clinical use of the tests. Results from ongoing testing in larger prospective clinical studies are also awaited. The added value of these tests for an optimised use of histopathology/immunohistochemistry (IHC) remains unclear at present. In a very recent study by Cuzick et al., a combined ER, PR, HER2 and Ki67 IHC score (IHC4), was shown to give similar prognostic information as the OncotypeDX in endocrine (only) treated patients [Citation53]. Also others have reported associations between several routine histopathological variables and the OncotypeDX Recurrence Score [Citation54,Citation55]. However, further comparisons of gene profiles, as MammaPrint and OncotypeDx [Citation56], with a selection of standard primary tumour analyses, are important in ongoing and new studies [Citation57], It might be that a combination of these analyses could be more accurate than strategies based on either set of parameters alone [Citation58,Citation59].
In conclusion, the results of the current study indicate that the combined analysis of VI, Grade, HER2 and Ki67 can be used for identification of an excellent prognosis group among node negative breast cancer patients with questionable adjuvant systemic treatment benefit. Such combined use of prognostic factors should be further tested and validated in larger clinical studies in parallel with new molecular multigene approaches.
Acknowledgements
We thank the staff at The Micrometastasis Laboratory, Department of Pathology, Radiumhospitalet, for their excellent technical assistance. We also thank statistician Milada Småstuen for statistical consultation. This study was supported by the Norwegian Research Foundation, Helse SørØst and Norwegian Cancer Society.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Early Breast Cancer Trialists’ Collaborative Group.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005;365:1687–717.
- Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989;63:181–7.
- Warwick J, Tabar L, Vitak B, Duffy SW. Time-dependent effects on survival in breast carcinoma: Results of 20 years of follow-up from the Swedish Two-County Study. Cancer 2004;100:1331–6.
- Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 2007;18:1133–44.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology, Breast Cancer, Version 2. 2011. Available from: http://www.nccn.com [cited 2012 January 17].
- Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 2009;20:1319–29.
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes – dealing with the diversity of breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011;22:1736–47.
- Norsk bryst cancer gruppe. Available from: http://www.nbcg.no. [cited 2012 January 17].
- Grogan M, Tabar L, Chua B, Chen HH, Boyages J. Estimating the benefits of adjuvant systemic therapy for women with early breast cancer. Br J Surg 2001;88:1513–8.
- Filho OM, Ignatiadis M, Sotiriou C. Genomic Grade Index: An important tool for assessing breast cancer tumor grade and prognosis. Crit Rev Oncol Hematol 2011;77:20–9.
- Oakman C, Bessi S, Zafarana E, Galardi F, Biganzoli L, Di LA. Recent advances in systemic therapy: New diagnostics and biological predictors of outcome in early breast cancer. Breast Cancer Res 2009;11:205.
- Parker JS, Mullins M, Cheang MC, Leung S, Voduc D, Vickery T, . Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol 2009;27:1160–7.
- Perou CM, Borresen-Dale AL. Systems biology and genomics of breast cancer. Cold Spring Harb Perspect Biol 2011;3.
- Slodkowska EA, Ross JS. MammaPrint 70-gene signature: Another milestone in personalized medical care for breast cancer patients. Expert Rev Mol Diagn 2009;9:417–22.
- Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med 2009;360:790–800.
- van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, . A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 2002;347: 1999–2009.
- Wirapati P, Sotiriou C, Kunkel S, Farmer P, Pradervand S, Haibe-Kains B, . Meta-analysis of gene expression profiles in breast cancer: Toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res 2008;10:R65.
- Naume B, Borgen E, Kvalheim G, Karesen R, Qvist H, Sauer T, . Detection of isolated tumor cells in bone marrow in early-stage breast carcinoma patients: Comparison with preoperative clinical parameters and primary tumor characteristics. Clin Cancer Res 2001;7:4122–9.
- Wiedswang G, Borgen E, Karesen R, Kvalheim G, Nesland JM, Qvist H, . Detection of isolated tumor cells in bone marrow is an independent prognostic factor in breast cancer. J Clin Oncol 2003;21:3469–78.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991;19:403–10.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol 2005;23:9067–72.
- Dowsett M, Nielsen TO, A’hern R, Bartlett J, Coombes RC, Cuzick J, . Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst 2011;103:1656–64.
- Jung SY, Han W, Lee JW, Ko E, Kim E, Yu JH, . Ki-67 expression gives additional prognostic information on St. Gallen 2007 and Adjuvant! Online risk categories in early breast cancer. Ann Surg Oncol 2009;16:1112–21.
- Chin SF, Daigo Y, Huang HE, Iyer NG, Callagy G, Kranjac T, . A simple and reliable pretreatment protocol facilitates fluorescent in situ hybridisation on tissue microarrays of paraffin wax embedded tumour samples. Mol Pathol 2003;56:275–9.
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, . American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med 2007;131:18–43.
- Azim HA, Jr., de AE, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol 2011;22:1939–47.
- Iqbal J, Ginsburg OM, Wijeratne TD, Howell A, Evans G, Sestak I, . Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: A systematic review. Cancer Treat Rev 2012;38:318–28.
- Zambetti M, Moliterni A, Materazzo C, Stefanelli M, Cipriani S, Valagussa P, . Long-term cardiac sequelae in operable breast cancer patients given adjuvant chemotherapy with or without doxorubicin and breast irradiation. J Clin Oncol 2001;19:37–43.
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, . Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011;378: 771–84.
- Grasic-Kuhar C, Bracko M, Zakotnik B. Risk factors for late relapse and death in patients with early breast cancer. Neoplasma 2008;55:416–20.
- Eden P, Ritz C, Rose C, Ferno M, Peterson C.“Good Old” clinical markers have similar power in breast cancer prognosis as microarray gene expression profilers. Eur J Cancer 2004;40:1837–41.
- Geradts J, Bean SM, Bentley RC, Barry WT. The oncotype DX recurrence score is correlated with a composite index including routinely reported pathobiologic features. Cancer Invest 2010;28:969–77.
- Lee AH, Ellis IO. The Nottingham prognostic index for invasive carcinoma of the breast. Pathol Oncol Res 2008;14:113–5.
- Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, . Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med 2010;7:e1000279.
- Christiansen P, Bjerre K, Ejlertsen B, Jensen MB, Rasmussen BB, Laenkholm AV, . Mortality rates among early-stage hormone receptor-positive breast cancer patients: A population-based cohort study in Denmark. J Natl Cancer Inst 2011;103:1363–72.
- Strand C, Ahlin C, Bendahl PO, Fjallskog ML, Hedenfalk I, Malmstrom P, . Combination of the proliferation marker cyclin A, histological grade, and estrogen receptor status in a new variable with high prognostic impact in breast cancer. Breast Cancer Res Treat 2012;131:33–40.
- Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, . Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst 2009;101: 736–50.
- de Azambuja E, Cardoso F, de CG, Jr., Colozza M, Mano MS, Durbecq V, . Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504–13.
- van Diest PJ, van der WE, Baak JP. Prognostic value of proliferation in invasive breast cancer: A review. J Clin Pathol 2004;57:675–81.
- Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol 2010;11:174–83.
- Trihia H, Murray S, Price K, Gelber RD, Golouh R, Goldhirsch A, . Ki-67 expression in breast carcinoma: Its association with grading systems, clinical parameters, and other prognostic factors – a surrogate marker?Cancer 2003;97:1321–31.
- Luporsi E, Andre F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, . Ki-67: Level of evidence and methodological considerations for its role in the clinical management of breast cancer: Analytical and critical review. Breast Cancer Res Treat 2012;132:895–915.
- Stuart-Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: A systematic review and meta-analysis of 85 studies in 32,825 patients. Breast 2008;17:323–34.
- Ruiz C, Seibt S, Al KK, Siraj AK, Mirlacher M, Schraml P, . Tissue microarrays for comparing molecular features with proliferation activity in breast cancer. Int J Cancer 2006;118:2190–4.
- Mohammed RA, Ellis IO, Mahmmod AM, Hawkes EC, Green AR, Rakha EA, . Lymphatic and blood vessels in basal and triple-negative breast cancers: Characteristics and prognostic significance. Mod Pathol 2011;24:774–85.
- Mohammed RA, Martin SG, Mahmmod AM, Macmillan RD, Green AR, Paish EC, . Objective assessment of lymphatic and blood vascular invasion in lymph node-negative breast carcinoma: Findings from a large case series with long-term follow-up. J Pathol 2011;223:358–65.
- Sabatier R, Jacquemier J, Bertucci F, Esterni B, Finetti P, Azario F, . Peritumoural vascular invasion: A major determinant of triple-negative breast cancer outcome. Eur J Cancer 2011;47:1537–45.
- Lee AH, Pinder SE, Macmillan RD, Mitchell M, Ellis IO, Elston CW, . Prognostic value of lymphovascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer 2006;42:357–62.
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol 2005;16:1569–83.
- Sloane JP, Amendoeira I, Apostolikas N, Bellocq JP, Bianchi S, Boecker W, . Consistency achieved by 23 European pathologists from 12 countries in diagnosing breast disease and reporting prognostic features of carcinomas. European Commission Working Group on Breast Screening Pathology. Virchows Arch 1999;434:3–10.
- Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, . Gene expression profiling in breast cancer: Understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst 2006;98:262–72.
- Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. Oncologist 2007; 12:631–5.
- Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, . Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the genomic health recurrence score in early breast cancer. J Clin Oncol 2011;29:4273–8.
- Auerbach J, Kim M, Fineberg S. Can features evaluated in the routine pathologic assessment of lymph node-negative estrogen receptor-positive stage I or II invasive breast cancer be used to predict the Oncotype DX recurrence score?Arch Pathol Lab Med 2010;134:1697–701.
- Flanagan MB, Dabbs DJ, Brufsky AM, Beriwal S, Bhargava R. Histopathologic variables predict Oncotype DX recurrence score. Mod Pathol 2008;21:1255–61.
- Kim C, Paik S. Gene-expression-based prognostic assays for breast cancer. Nat Rev Clin Oncol 2010;7:340–7.
- Mehta R, Jain RK, Badve S. Personalized medicine: The road ahead. Clin Breast Cancer 2011;11:20–6.
- Parisi F, Gonzalez AM, Nadler Y, Camp RL, Rimm DL, Kluger HM, . Benefits of biomarker selection and clinico-pathological covariate inclusion in breast cancer prognostic models. Breast Cancer Res 2010;12:R66.
- Zhao X, Rodland EA, Sorlie T, Naume B, Langerod A, Frigessi A, . Combining gene signatures improves prediction of breast cancer survival. PLoS One 2011;6: e17845.