Abstract
Objective. Metastatic seminoma is a highly curable disease. Standard treatment comprises of combination chemotherapy. The short- and long-term toxicities of this treatment are increasingly recognised and the possibility of over treatment in such a curable disease should be considered. We have therefore assessed the use of single agent carboplatin at a dose of AUC 10 in patients with good prognosis metastatic seminoma. Materials and methods. Patients with good prognosis metastatic seminoma treated with carboplatin (AUC 10) were identified at our institution and affiliated institutions. Treatment was three weekly for a total of three or four cycles. Outcome and toxicities were analysed. Results. With a median follow-up of 36 months, 61 patients in total were treated with carboplatin AUC 10, all good prognosis by the IGCCCG criteria. Forty-eight percent had stage IIA/IIB disease and 52% had greater than stage IIB disease. Thirty-one patients (51%) had a complete response following treatment. Three-year survival was 96.3% with a three-year progression free survival of 93.2%. The main treatment toxicity was haematological with 46% having grade 3, 24% having grade 4 neutropenia and 54% experiencing grade 3/4 thrombocytopenia. There were no treatment related deaths. Conclusion. Single agent carboplatin at a dose of AUC 10 is an effective treatment for good prognosis metastatic seminoma. The outcome compares favourably to previously published outcomes of combination chemotherapy. Although haematological toxicity is a concern, single agent carboplatin treatment for good prognosis metastatic seminoma could be considered a treatment option and is associated with less toxicity than combination regimens currently used.
Germ cell tumours are highly treatable malignancies even in the metastatic setting [Citation1]. It has long been known that seminomas are more radiosensitive than non-seminomatous germ cell tumours (NSGCTs) [Citation2]. The precise degree, however, is hard to establish from previous published series of metastatic seminoma, as non-seminoma has a higher propensity to metastasise. Less work has been done on the relative chemosensitivity of seminoma and non-seminoma. A small phase II study, published in 1984, of single agent cisplatin in metastatic seminoma reported 81% relapse free survival [Citation3] suggesting exquisite sensitivity of seminomas to platinum complexes. Early studies with carboplatin showed that it was also a highly potent drug in metastatic seminoma [Citation4,Citation5]. Two randomised trials, however, with a total of 361 patients comparing single agent carboplatin, at a dose of 400 mg/m2 (actual dose adjusted according to creatinine clearance) with cisplatin combination regimes showed the progression free survival to be significantly inferior for the single agent. After a median follow-up of 4.5 years results reported a borderline but non-significant difference in overall survival of 5% in favour of the combination group [Citation6]. A consequence of these two trials – and other trials in NSGCT – was that three cycles of the cisplatin combination regimen, BEP (cisplatin, etoposide and bleomycin) chemotherapy has become the standard treatment for patients with good prognosis metastatic germ cell tumours including seminoma.
It is, however, questionable whether the dose of carboplatin used in these randomised studies was optimal. In older ovarian cancer patients a dose up to AUC 12 was tolerated without bone marrow support [Citation7]. Our previous studies also suggest that there could be a dose-response effect [Citation8].
The difference in short-term toxicity between BEP and single agent carboplatin is considerable. Furthermore, there is also the problem of the long-term toxicity of BEP chemotherapy which one particularly wishes to avoid in young patients with a curable disease. This toxicity relates to a range of cardiovascular, endocrine, auditory and neurological sequelae as well as the potential for second malignancies [Citation9–12]. Although there is less data on the long-term toxicity of carboplatin, in comparison to cisplatin, carboplatin has been shown to cause less nausea, emesis, renal toxicity, peripheral neuropathy and ototoxicity [Citation9,Citation13,Citation14], making it an attractive chemotherapy option.
Nine years ago we began a formal phase II study investigating carboplatin AUC 10 every 21days in IGCCCG good prognosis metastatic seminoma patients [Citation15]. The results from this phase II was reported in 2010 [Citation16] showing the dose was tolerable and effective. The reassuring results from the phase II and the outcome of patients treated prior to that made this collaborative group feel confident to offer this as a treatment option in the management of IGCCG good prognosis seminoma. We now report the updated results.
Methods
Eligibility
Between 1997 and 2010 all patients with a diagnosis of good prognosis metastatic seminoma who received carboplatin AUC 10 were identified across treatment centres. Included in the analysis are the updated results from the phase II trial and patients who have received carboplatin since the trial. A small number of patients received single agent carboplatin prior to the formal phase II trial and we have included data on these patients.
All patient data were collected retrospectively and disease was staged according to The American Joint Committee on Cancer (2010 edition) TMN classification [Citation17] with imaging of the chest abdomen and pelvis and serum tumour markers: β-human chorionic gonadotrophin (βHCG), α fetoprotein (AFP) and lactate dehydrogenase (LDH). All patients had a diagnosis of good prognosis metastatic seminoma as determined by the International Germ Cell Cancer Collaborative Group (IGCCCG) criteria. Patients were ineligible if they had received prior chemotherapy treatment or radiation treatment for stage I disease.
Treatment
Carboplatin was dosed at AUC 10 based on the Calvert formula {total dose (mg) = target AUC × [GFR (ml/min) + 25]}. Glomerular filtration rate (GFR) was measured by a formal EDTA clearance result or if that was not available the dose was based on a GFR calculated by the Cockcroft-Gault equation. There was no upper dose limit. The treatment was administered as an one-hour outpatient treatment once every 21 days for three or four cycles.
Dose modifications
If a complete response was established based on imaging and tumour markers after cycle 1, three cycles of carboplatin were administered in total. If there was stable disease/partial response following cycle 1, four cycles of carboplatin were given. If the platelet nadir was less than 20 then a 20% dose reduction was administered on subsequent cycles. If a patient was admitted with neutropenia prophylactic antibiotics with or without filgrastim were used on subsequent occasions. Blood products were administered as required according to local protocols. The carboplatin dose was based on creatinine clearance at the start of the treatment and was not recalculated based on creatinine changes each cycle.
Evaluation
All patients had a pretreatment full blood count (FBC), urea and electrolytes (U&E), liver function tests (LFT), LDH, AFP and HCG prior to each cycle. A nadir blood count was checked after each cycle at days 10–14. A whole body PET-CT (if available) or CT-scan of the abdomen, chest and pelvis was performed prior to treatment, prior to cycle 2 and at the end of treatment. Evaluation of response to chemotherapy was determined by imaging and serum tumour markers. Complete response (CR) was defined as resolution of disease on imaging (short axis of lymph nodes under 1 cm) and normalisation of serum tumour markers. Marker-negative partial response (M-PR) was defined as the presence of a residual mass following treatment but with normalisation of serum tumour markers. If serum tumour markers were not elevated prior to treatment, partial response was determined according to Response Evaluation Criteria in Solid Tumours (RECIST) criteria. Progressive disease was determined by a combination of progression of disease on imaging and/or a rise in serum tumour markers.
All toxicities were registered according to the Common Terminology Criteria for Adverse Events Version 4.0 Cancer Evaluation program, NCI. Toxicities were recorded at time of treatment but data has subsequently been collected retrospectively.
Follow-up
Patients were followed up every two months during the first year, every four months during the second year, six monthly during the third year, and once yearly thereafter until death. Follow-up investigations included baseline haematological and biochemical investigations and tumour markers (HCG, AFP and LDH) at each visit. A further CT-scan was performed at one and two years post completion of chemotherapy.
Patients with residual disease of ≥3 cm after a period of observation were advised to proceed to retroperitoneal lymph node dissection (RPLND) in line with current practices. Following surgery a repeat CT-scan was performed at 2–3 months and if complete response was achieved by chemotherapy with or without surgery a final CT-scan was performed at two years.
Statistical analysis
Progression free survival and overall survival was defined in months after the first day of treatment until the last follow-up. Survival estimates were calculated using the Kaplan-Meier analysis.
Results
Patient characteristics
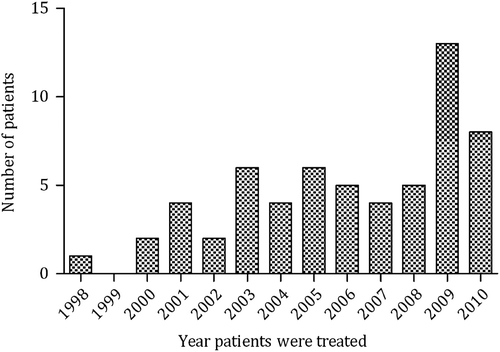
Sixty-one patients were treated with single agent carboplatin AUC 10 (date patients treated shown in ). All patients were good prognosis by the IGCCCG criteria, 46% stage IIA/IIB disease and 54% had greater than stage IIB disease. Median age was 38 (range 24–67) (baseline characteristics shown in ). Forty-seven patients (77%) had a formal EDTA to calculate their creatinine clearance. The main explanation for no formal EDTA was that it was not possible to perform the test in the timescale or the test was not available. Average dose of carboplatin per cycle was 1438 mg (approximately equal to 700 mg/m2), (range 840–2250 mg).
Table I. Baseline characteristics.
Response to treatment
Twenty patients had a complete response (CR) after one cycle of treatment and went on to receive three cycles of carboplatin. Forty-one patients had a partial response (PR) or stable disease (SD) following one cycle and received four cycles of treatment. One patient had a PR after two cycles and then went abroad and was lost to follow-up. Thirty-one patients (51%) had a CR at the end of treatment scan. Twenty-seven patients (44%) had a marker negative partial response (M-PR) following treatment. Responses to chemotherapy are listed in .
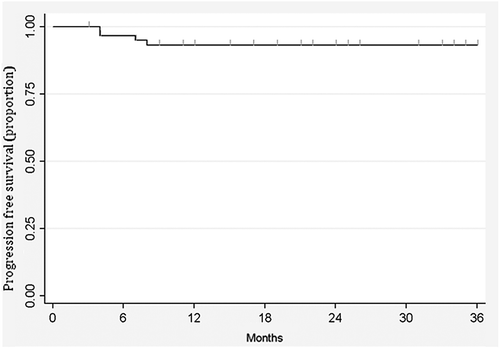
After a median follow-up of 36 months, three-year overall survival was 96.2% (CI 85.6–99) and progression free survival 93.2% (CI 82.8–97.4) (Kaplan Meier curve, ).
Table II. Response of patients according to stage following treatment with single agent carboplatin.
During follow-up three patients relapsed, two of these patients had stage IIIB and one stage IIC disease at diagnosis. All relapses had four cycles of carboplatin, two achieved a marker negative partial response and all relapses occurred within seven months after initial treatment. One patient had a partial response on the scan following his first chemotherapy cycle but progressed on his end of treatment scan. He went on to progress through BEP (bleomycin, etoposide and cisplatin) chemotherapy and died 13 months after diagnosis. Two of the three relapses, however, were salvaged successfully with BEP and remain disease free ().
Table III. Details of patients who relapsed following carboplatin treatment.
One patient required a retroperitoneal lymph node dissection following treatment due to residual disease. No evidence of malignancy was seen at histology.
Toxicity
Haematological toxicity data is shown in and . Due to retrospective collection of results and treatment in multiple different centres data was not available for seven patients. Results are all calculated as percentage of available data. Though overall 70% experienced an episode of grade 3/4 neutropenia only one patient was admitted for febrile neutropenia. Dosage adjustment according to protocol and prophylactic filgrastim as per the local guidelines in 10 patients resulted in good subsequent tolerance. In total 23% of chemotherapy cycles (30% of patients) required prophylactic filgrastim.
Table IV. Haematological toxicities (NCI Common Terminology Criteria for Adverse Events version V4.0).
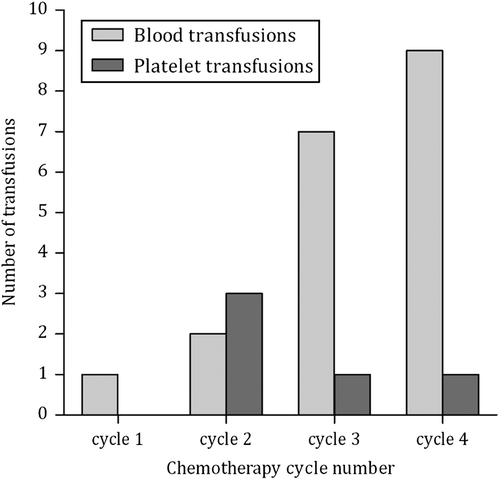
Fifty-four percent experienced an episode of grade 3 or 4 thrombocytopenia, dosage adjustment according to protocol resulted in good subsequent tolerance. Overall 26% experienced an episode of grade 3 or 4 anaemia. There were two admissions for nausea and vomiting. In total 17 patients required blood product support (28%) () with seven patients requiring both blood and platelet transfusions or repeat transfusions on subsequent cycles. Overall, the regime was well tolerated by the majority of patients with 72% not requiring any blood product support and 70% did not require filgrastim support.
Non-haemtological toxicity
Minimal changes in renal function were seen during treatment and no patient required dose adjustments according to renal function. Fifty percent of patients experienced a grade 1 creatinine rise by the end of treatment but in all these patients the change was less than 20% and in the majority (76%) the creatinine increased by less than 10%. Only one patient experienced a grade 2 rise in creatinine which has improved with subsequent follow-up. No patient experienced hair loss.
Discussion
Metastatic seminoma is a highly curable disease. The ideal treatment not only needs to cure the disease but also needs to focus on reducing long- and short-term toxicities. The standard treatment is currently combination chemotherapy, however, long-term toxicities are increasingly being recognised. We have therefore investigated the use of single agent carboplatin in this group of patients.
The data presented here shows that treatment of good prognosis metastatic seminoma with carboplatin AUC 10 is effective and safe. Our data shows three year progression free survival of 93.2% and overall survival of 96.2%. Our patient population also included a significant proportion with advanced disease at presentation with 53% having greater than stage IIB disease. There were no relapses in patients with stage IIA/B disease but three relapses of the 33 (9%) patients with greater than stage IIB disease. This compares favourably with previous results using cisplatin based combination regimens for the treatment of metastatic seminoma. The Spanish Germ Cell Cancer Group [Citation18] reported a five year progression free survival of 90% (95% CI 82–98%) and overall survival of 95% (95% CI 89–100%). Our results also compare well with two recent population-based studies from the Swedish Norwegian Testicular Cancer Study Group [Citation19] and the combined data from the British Columbia Cancer Agency (BCCA) and Oregon Testis Cancer Program [Citation20]. The Swedish Norwegian Testicular Cancer Study Group reported no relapses in patients with stage IIA/B disease following treatment with chemotherapy (but three relapses of the 29 patients treated with radiotherapy). Six of the 42 patients (14.9%) with stage IIC disease relapsed after treatment with combination cisplatin chemotherapy and there was one patient death. The BCCA and Oregon Testis Cancer Program reported a five year actuarial relapse-free survival rate in patients with stage II seminomas of 95.5% following combination chemotherapy. Our results appear superior to those reported using carboplatin AUC 7 when given every 28 days instead of 21 [Citation21] which reported an overall failure rate in 19 of 108 (18%) patients with stage IIA/B disease. Our results also appear better than the carboplatin arm of the pooled analysis of two randomised European trials using carboplatin 400 mg/m2 which reported a five year overall survival rate of 89% and progression free survival rate of 72% [Citation6]. In addition, the results from this study are at least as good as carboplatin and radiation for stage IIB patients [Citation22] and possibly less risky given that previous studies of late events have shown that it is combined chemotherapy and radiation that has the greatest risk of secondary non-germ cell malignancy [Citation23].
Haematological toxicity is a concern due to the need for blood products; however, giving three courses of treatment for all would probably have achieved a better therapeutic ratio giving less toxicity and a better chance for salvage therapy to cure resistant tumours. Increased use of PET-CT to establish those in metabolic CR following cycle 1 [Citation8] may further assist to limit treatment to three cycles. Even so the toxicity is less than that of combination regimes or radiotherapy [Citation24,Citation25].
Carboplatin is given as an one-hour outpatient treatment once every 21 days for three or four courses compared with the standard combination regime that is administered as a 3–5 day 8 hour in/day-patient infusion every 21 days with outpatient chemotherapy Day 8 and 15 for three courses. Given the growing need for cost containment of healthcare costs [Citation26] single agent carboplatin may offer a more economical option and offer patient choice of an outpatient treatment given just on one day every three weeks.
Although this study is limited by being non- randomised and further follow-up is required, our results suggest that single agent carboplatin has a role in the treatment of good prognosis metastatic seminoma. We feel that single agent carboplatin should be reinvestigated in this group of patients. This should be a prospective study, either a randomised phase III study, or a population based cohort study with enough patients to ensure mature follow-up.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Einhorn LH. Treatment of testicular cancer: A new and improved model. J Clin Oncol 1990;8:1777–81.
- Friedman N. Supervoltage (1 million volts) roentgen therapy at Walter Reed General Hospital. Surg Clin N Am 1944; 24:1424–32.
- Oliver RTD, Hope-Stone HF, Blandy JP. Possible new approaches to the management of seminoma of the testis. Br J Urol 1984;56:729–33.
- Horwich A, Dearnaley DP, A’Hern R, Mason M, Thomas G, Jay G, et al. The activity of single-agent carboplatin in advanced seminoma. Eur J Cancer 1992;28A:1307–10.
- Oliver RT, Lore S, Ong J. Alternatives to radiotherapy in the management of seminoma. Br J Urol 1990;65:61–7.
- Bokemeyer C, Kollmannsberger C, Stenning S, Hartmann JT, Horwich A, Clemm C, et al. Metastatic seminoma treated with either single agent carboplatin or cisplatin-based combination chemotherapy: A pooled analysis of two randomised trials. Br J Cancer 2004;91:683–7.
- Gore M, Mainwaring P, A’Hern R, MacFarlane V, Slevin M, Harper P, et al. Randomized trial of dose-intensity with single-agent carboplatin in patients with epithelial ovarian cancer. London Gynaecological Oncology Group. J Clin Oncol 1998;16:2426–34.
- Oliver R, Powles T, Ell P, Somasundram U, Shamash J. 22 year phase study of single agent carboplatin in metastatic seminoma: Potential for acceleration by a new surrogate end point, 72 hr PET scan response?J Clin Oncol (Meeting Abstracts) 2006;24:14565.
- Glendenning JL, Barbachano Y, Norman AR, Dearnaley DP, Horwich A, Huddart RA. Long-term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer 2010;116:2322–31.
- Bokemeyer C, Berger CC, Kuczyk MA, Schmoll HJ. Evaluation of long-term toxicity after chemotherapy for testicular cancer. J Clin Oncol 1996;14:2923–32.
- Fossa SD, Gilbert E, Dores GM, Chen J, McGlynn KA, Schonfeld S, et al. Noncancer causes of death in survivors of testicular cancer. J Natl Cancer Inst 2007;99:533–44.
- Fossa SD, de Wit R, Roberts JT, Wilkinson PM, de Mulder PH, Mead GM, et al. Quality of life in good prognosis patients with metastatic germ cell cancer: A prospective study of the European Organization for Research and Treatment of Cancer Genitourinary Group/Medical Research Council Testicular Cancer Study Group (30941/TE20). J Clin Oncol 2003;21:1107–18.
- Powles T, Robinson D, Shamash J, Moller H, Tranter N, Oliver T. The long-term risks of adjuvant carboplatin treatment for stage I seminoma of the testis. Ann Oncol 2008;19:443–7.
- Sakaeda T, Kadoyama K, Okuno Y. Adverse event profiles of platinum agents: Data mining of the public version of the FDA adverse event reporting system, AERS, and reproducibility of clinical observations. Int J Med Sci 2011;8:487–91.
- International Germ Cell Consensus Classification:A prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol 1997;15:594–603.
- Matakidou A, Mutsvangwa K, Ansell W, Lim L, Powles TB, Oliver RT, et al. Single-agent carboplatin AUC 10 for metastatic seminoma with IGCCCG good prognosis disease; a feasibility study of the Orchid Clinical Trials Group. Ann Oncol 2010;21:1730–1.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A editors. AJCC cancer staging manual, 7th ed. New York, NY: Springer; 2010.
- Garcia-del-Muro X, Maroto P, Guma J, Sastre J, Lopez Brea M, Arranz JA, et al. Chemotherapy as an alternative to radiotherapy in the treatment of stage IIA and IIB testicular seminoma: A Spanish Germ Cell Cancer Group Study. J Clin Oncol 2008;26:5416–21.
- Tandstad T, Smaaland R, Solberg A, Bremnes RM, Langberg CW, Laurell A, et al. Management of seminomatous testicular cancer: A binational prospective population-based study from the Swedish Norwegian Testicular Cancer Study Group. J Clin Oncol 2011;29:719–25.
- Kollmannsberger C, Tyldesley S, Moore C, Chi KN, Murray N, Daneshmand S, et al. Evolution in management of testicular seminoma: Population-based outcomes with selective utilization of active therapies. Ann Oncol 2011; 22:808–14.
- Krege S, Boergermann C, Baschek R, Hinke A, Pottek T, Kliesch S, et al. Single agent carboplatin for CS IIA/B testicular seminoma. A phase II study of the German Testicular Cancer Study Group (GTCSG). Ann Oncol 2006;17:276–80.
- Gilbert DC, Vanas NJ, Beesley S, Bloomfield D, Money-Kyrle J, Norman A, et al. Treating IIA/B seminoma with combination carboplatin and radiotherapy. J Clin Oncol 2009;27:2101–2; author reply 2–3.
- Travis LB, Fossa SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, et al. Second cancers among 40,576 testicular cancer patients: Focus on long-term survivors. J Natl Cancer Inst 2005;97:1354–65.
- Horwich A, Oliver RT, Wilkinson PM, Mead GM, Harland SJ, Cullen MH, et al. A medical research council randomized trial of single agent carboplatin versus etoposide and cisplatin for advanced metastatic seminoma. MRC Testicular Tumour Working Party. Br J Cancer 2000; 83:1623–9.
- Fossa SD, Langmark F, Aass N, Andersen A, Lothe R, Borresen AL. Second non-germ cell malignancies after radiotherapy of testicular cancer with or without chemotherapy. Br J Cancer 1990;61:639–43.
- Bernard DS, Farr SL, Fang Z. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J Clin Oncol 2011;29:2821–6.