Abstract
Background. Adult Langerhans cell histiocytosis is a rare disorder with diverse clinical manifestations and inconsistent treatment outcomes to conventional therapeutic regimens. Cladribine (2-chlorodeoxyadenosine) repeatedly proved effective in cases of relapsed multifocal and multisystem disease forms. In this retrospective study we present an analysis of cladribine in frontline systemic therapy. Material and methods. A cohort of seven male patients with biopsy proved multisystem (six cases) and multifocal (one case) Langerhans cell histiocytosis received cladribine at a dose of 5 mg/m2 subcutaneously (five cases) or by two-hour intravenous infusion (two cases) over five consecutive days, every four weeks for a median of four courses (range 4–6). The treatment was enhanced with cyclophosphamide (300 mg intravenously on days 1–5 in cycles 4–6) and corticoids (dexamethasone 24 mg orally or methylprednisolone 250 mg intravenously on days 1–5 in cycles 4–6) in two patients, with radiotherapy (20 Gy on skin or bone lesions) in three patients and with photochemotherapy (psoralen plus ultraviolet A light, PUVA) on skin lesions in one patient. Results. All patients achieved clinically relevant treatment response confirmed by positron emission tomography (PET). Durable complete remissions were maintained in six patients (86%), including two patients with hypophysis involvement, with the median follow-up of 37 months (range 15–94; 49.8 ± 35.2 [Citation]). One patient had an aggressive, early relapsing disease requiring further treatment lines. The treatment-related toxicities consisted of transient bone marrow suppression affecting the leukocytes predominantly. Grade 3 lymphopenia occurred in five patients (71%) and grade 3 neutropenia in one patient (14%). Conclusion. Cladribine, both as a single agent as well as in combination with an alkylating cytostatic and corticoids, represents an effective treatment option with favorable toxicity profile for adult patients with multisystem or aggressive multifocal form of Langerhans cell histiocytosis.
Langerhans cell histiocytosis (LCH) is a rare idiopathic disorder characterized by dysregulated proliferation and accumulation of pathological Langerhans cells in various tissues and organs [Citation1]. Though occurring at all ages, it mostly arises in children aged one to three years. The clinical manifestation is diverse ranging from a single osteolytic lesion (eosinophilic granuloma) to widespread disease with multiorgan involvement. Equally variable is the natural history of the disease with cases of spontaneous remission on one side and rapid progression or multiple recurrences on the other [Citation2].
According to the Histiocyte Society Treatment Protocol for LCH, the disease is classified into two main groups, i.e. single system disease further divided into unifocal and multifocal forms, and multisystem disease. This differentiation is reflected in the therapeutical approach, where local therapies are applied in unifocal LCH, while systemic treatment is required in multifocal and multisystem diseases. Moreover, pediatric patients with organ dysfunctions in the multisystem LCH category are subclassified into low-risk (skin, bone, lymph node, pituitary) and high-risk (lung, liver, spleen, bone marrow) groups [Citation3,Citation4]. In adults however, no risk organs associated with poor prognosis have been defined so far. This is due to low incidence of LCH in adults, which is estimated at one to two cases per million per year [Citation5,Citation6]. Nevertheless, similarly to children, multisystem involvement and early disease relapse after therapy signalize less favorable outcome [Citation6].
For multisystem disease various treatment regimens including glucocorticoids, immunomodulation, chemotherapy, radiotherapy and peripheral blood stem cell transplantation have been proposed but the lack of controlled clinical trials for adult patients proved to be limiting in making firm therapy recommendations [Citation7]. Therefore, therapeutic protocols are often derived from experience with pediatric LCH. However, vinblastine that is still considered gold standard in the management of LCH in children, may exhibit severe neurotoxic effects in adults [Citation8] and concomitant prednisone administration may increase risk for diabetes mellitus [Citation9]. Long therapy duration [Citation10] and potential soft tissue toxicity of vinblastine in case of extravasation [Citation11] further contribute to reconsideration of its role in management of LCH in adults.
Triggered by the first successful treatment in 1993 [Citation12], cladribine gained popularity in management of relapsed multifocal and multisystem LCH. Published single case reports and small series studies demonstrated encouraging response rates with a favorable toxicity profile in both pediatric and adult LCH [Citation13–18]. An April 2012 MEDLINE literature search, using the keywords Langerhans cell histiocytosis, adult, cladribine, 2-chlorodeoxyadenosine, revealed three adult studies with at least five patients included, all of which dealing with relapsed or refractory cases [Citation15–17]. In this work we retrospectively studied a group of seven patients with biopsy proved LCH and we present here excellent results of the largest analysis of cladribine treatment in adult LCH patients as the initial systemic therapy.
Materials and methods
Patients
During a 22-year period from November 1, 1989, to January 1, 2012, 23 patients were diagnosed with Langerhans cell histiocytosis and followed up at our department. Of those, nine patients (eight males and one female) were treated with cladribine. A cohort of seven male patients, who had received cladribine as frontline therapy (between 2003 and 2010), was retrospectively analyzed and included in our study. The diagnosis of LCH was established based on histological and immunophenotypic examinations of lesional cells (). Patients who did not agree to the use of their medical records for research were not included in the study.
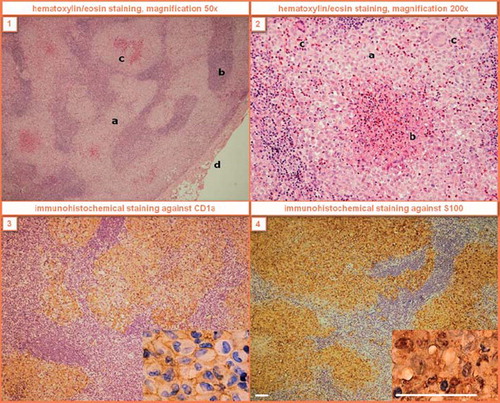
Figure 1. Histological findings of LCH in the right supraclavicular lymph node of Case 6. (1) The node is extensively infiltrated by coalescent, focally necrotized infiltrates with prevailing sinusoidal distribution (a – LCH infiltrates, b – lymph node tissue, c – necrotic foci, d – lymph node capsule). (2) The lesional cells have bland lobular nuclei frequently with indentations and abundant cytoplasm (a – Langerhans cells with reactive eosinophils, b – eosinophilic abscess with necrosis, c – giant multinucleated cells). The immunohistochemical staining causes brown membrane (3) and brown nuclear and cytoplasmatic (4) colorings. White scale line = 50 μm.
Chemotherapy
Cladribine was administered as subcutaneous injection (five patients) or by two-hour intravenous infusion (two patients) at a dose of 5 mg/m2 over five consecutive days in four week intervals for a median of four cycles (range 4–6 cycles; 4.9 ± 1.1 [Citation7]). In two patients, aggressive disease courses (rapid progression, excessive disease extent or presence of B-symptoms) were observed making them potential candidates for high-dose chemotherapy with autologous peripheral blood stem cell transplantation. Therefore, they underwent peripheral blood stem cell mobilization after stimulation regimens with cyclophosphamide (2 g/m2 intravenous on day 1) and etoposide (200 mg/m2 intravenous on days 1–3) followed by filgrastim application (10 μg/kg per day subcutaneously) prior to cladribine application. The first patient (Case 2) was successfully treated with cladribine and did not require any further chemotherapy, in the second male (Case 6) a refractory LCH was the case that indicated the patient for two more treatment lines including autologous as well as allogeneic blood stem cell transplantations. The latter case had been published previously [Citation19].
Cladribine treatment response evaluation was performed after the 2nd or 3rd cycle. In two cases (4 and 6) with insufficient therapy results, cladribine monotherapy was enhanced by corticoids (dexamethasone 24 mg orally or methylprednisolone 250 mg intravenously on days 1–5 in cycles 4–6) and cyclophosphamide (300 mg intravenously on days 1–5 in cycles 4–6).
Other treatment modalities
All patients were administered cladribine as frontline chemotherapy. Concomitant radiotherapy was applied in Cases 3, 5 and 6. PUVA photochemotherapy (psoralen plus ultraviolet A light) on residual dermal lesions was used in Case 4. In Case 1, ostelytic lesions in the 12th thoracic vertebrae and right femoral bone had initially been treated surgically using allografts and after 10 years, when the disease relapsed with multiple bone and newly also lung involvements, the patient was indicated for systemic chemotherapy with cladribine.
Treatment response evaluation
In selected patients, apart from clinical investigations and laboratory (endocrinological) testing, radiological [bone x-ray, computed tomography (CT), magnetic resonance imaging (MRI)] and nuclear medicine modalities [traditional bone scintigraphy, positron emission tomography (PET)] as well as pulmonary function tests were carried out. PET alone or especially in combination with CT imaging were the main decisive examinations applied in all patients. In our study, complete remission represents total retreat of disease activity confirmed by a negative finding on PET/CT.
In two patients with central diabetes insipidus, pituitary stalk infiltrations were detected on MRI. To disclose potential extracranial manifestations of an unknown disease, PET/CT examinations were performed showing increased tracer uptake in the perianal area (SUVmax = 8.6) in Case 3 and cystic pulmonary lesions in combination with nodularities in Case 7. Based on a perianal skin biopsy and a bronchoalveolar lavage, respectively, LCH was diagnosed in both patients. Subsequently, regressions of the hypophyseal infiltrates on restaging MRI during cladribine treatments confirmed the presumed central form of LCH, thus avoiding unnecessary pituitary biopsies.
Results
Seven males, from 21 to 46 years of age at the time of LCH diagnosis (35 ± 8.5 [Citation7]), were included in this study. Six patients had multisystem disease and one patient (Case 2) was diagnosed with multifocal bone LCH (, ). In the cohort, the following organs were affected: the lungs (71%), bones (43%), ear (29%), gingiva (29%), hypophysis (29%), skin (29%) and lymph nodes (14%).
Figure 2. T1 weighted MRI scans of the brain in transverse (left) and sagittal (right) planes after application of contrast agent in Case 2. Due to incipient eyesight deterioration and increased tracer uptake in the occipital region on bone scintigraphy, the patient was sent to MRI which revealed an osteolytic lesion infiltrating the meningeal membranes near the occipital lobe. The function of the centre of vision which is located in the occipital lobe was deteriorated in this patient by intracranial propagation of LCH. After cladribine treatment the infiltration receded, which was followed by a complete vision recovery.
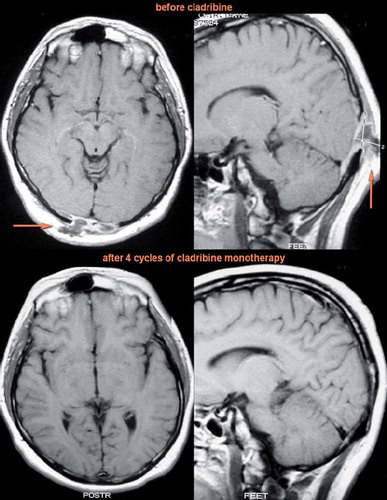
Table I. Main demographic and clinical characteristics of seven patients treated with cladribine.
In several weeks after the diagnosis of LCH had been confirmed, the patients were started on cladribine except for Case 1 which had initially been treated surgically. In two patients, longer times to cladribine start were due to initial radiotherapy preceding chemotherapy (Case 5 delayed for three months) and a wish to ground a family (Case 7 delayed for nine months). All patients achieved clinically relevant treatment response confirmed by PET/CT examination (). Durable complete remissions were maintained in six of seven patients (86%) with the median follow-up of 37 months (range 15–94; 49.8 ± 35.2 [Citation6]). In Case 6, the disease relapsed in two months after the last course of cladribine.
Table II. An overview of cladribine-based treatment and therapy responses in seven adult patients with LCH.
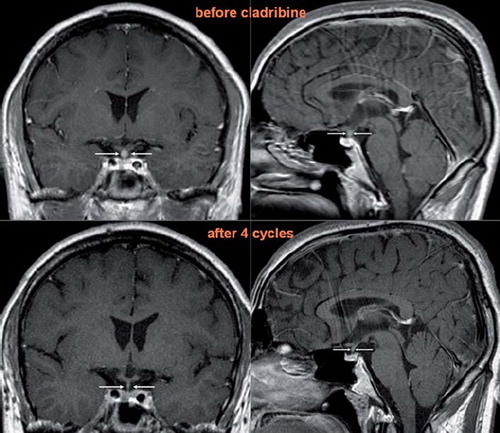
Disappearance of pituitary infiltrate was observed in both patients with LCH affecting the hypophyseal infundibulum. In Case 3, restaging MRI after the 4th cycle revealed complete remission of the pituitary infiltration (). In Case 7, the MRI after the 4th cycle showed partial regression in size of the infiltrate which disappeared fully after the 6th cycle. However, both patients continue in their hormone replacement therapies.
Figure 3. T1 weighted MRI scans of the brain in coronary (left) and sagittal (right) planes after application of contrast agent in Case 3. The ball-shaped enlargement (infiltration of the hypophysis infundibulum by LCH) fully retreated after cladribine monotherapy.
Two patients (Cases 4 and 6) required treatment intensification after three courses of cladribine monotherapy. In Case 4 with pulmonary and cutaneous involvements, three cycles of cladribine monotherapy only led to a partial reduction of the skin lesions but not to remission. Therefore, cyclophosphamide and dexamethasone were added from the 4th cycle on. Since residual dermal affection was still visible after two cycles of the combination regimen, PUVA photochemotherapy was used. The skin symptoms then remitted completely after completion of chemotherapy and PUVA treatment. In Case 6 with pulmonary, cutaneous and lymph node involvements, perianal skin pruritus with local induration and right axillary lymph node enlargement persisted after three courses of cladribine monotherapy. Consequently, from the 4th cycle on the patient was administered three cycles of combined chemotherapy (cladribine, cyclophosphamide and dexamethasone) completed with curative radiotherapy of the perianal area.
Treatment toxicities
Hematological toxicities are summarized in . The median leukocyte count, hemoglobin level and thrombocyte count at the time of therapy initiation were 7.4 × 109 cells/l (range 4.7–8.4; 6.9 ± 1.4 [Citation7]), 147 g/l (range 125–160; 143.9 ± 12.8 [Citation7]) and 247 × 109 cells/l (range 217–589; 313.6 ± 131.3 [Citation7]), respectively. The median leukocyte count, hemoglobin level and thrombocyte count at the beginning of the last therapy cycle were 5.7 × 109 cells/l (range 3.8–6.7; 5.6 ± 1.0 [Citation7]), 142 g/l (range 129–166; 144.6 ± 15.4 [Citation7]) and 204 × 109 cells/l (range 134–346; 211.3 ± 65.7 [Citation7]), respectively. The median lowest leukocyte count, hemoglobin level and thrombocyte count during the treatment were 3.8 × 109 cells/l (range 1.6–5.7; 3.9 ± 1.4 [Citation7]), 129 g/l (range 125–153; 134.4 ± 11.4 [Citation7]) and 167 × 109 cells/l (range 134–276; 178.4 ± 46.3 [Citation7]), respectively.
Table III. Hematological treatment-related toxicities during the cladribine-based treatment.
No significant anemia or thrombocytopenia was observed in any of the patients. In Case 2 transient leukopenia grade 3 (1.6 × 109 cells/l) with neutropenia grade 3 (0.5 × 109 cells/l) and lymphopenia grade 3 (0.4 × 109 cells/l) were complicated by shingles eruption resulting in one-week hospitalization. In Case 4, mild tonsillitis required peroral antibiotics due to ongoing chemotherapy. Cases 3, 6 and 7 experienced transient isolated lymphopenias grade 3 with asymptomatic courses. Despite the well known immunosuppressive effect of cladribine, no other infectious intercurrences were documented in our cohort. Radiation dermatitis grade 3 or 4 was not reported likewise.
Discussion
Cladribine (2-chlorodeoxyadenosine) entered clinical practice in the early 1990s. It is an adenosine derivative with hydrogen substituted for chlorine in the second carbon of the purine ring. This change is sufficient to prevent deamination of 2-chloroadenosine triphosphate, i.e. the effective compound itself. 2-chloroadenosine triphosphate tends to accumulate in the cells whose activating (phosphorylation) enzyme, deoxycytidine kinase, is the most active, while the inactivating (dephosphorylation) enzyme, cytoplasmic 5’-nucleotidase, is the least active. In resting and proliferating lymphocytes, monocytes, histiocytes and also Langerhans cells, a very favorable ratio of these two enzymes for the effectiveness of cladribine can be observed. In these cells intracellular concentrations of 2- chloroadenosine triphosphate are several hundred times above plasmatic concentrations [Citation20,Citation21].
Thanks to the aforementioned characteristics, cladribine is a highly selective cytostatic agent with intense cytotoxic effects on slowly progressing lymphoid malignancies (chronic lymphocytic leukemia, hairy cell leukemia, Waldenström's macroglobulinemia) as well as histiocytic disorders (LCH, Erdheim-Chester disease, Rosai-Dorfman disease). In contrast to vinblastine, cladribine penetrates into the intrathecal space in effective amounts and thus possesses a treatment potential in patients with central nervous system involvement [Citation22]. Bioavailability of cladribine in the central nervous system and spinal fluid after subcutaneous administration is 100% and thus both intravenous and subcutaneous administration is possible. Pharmacological properties of cladribine (100% absorption from subcutaneous tissue, several hundred-fold higher accumulation in the target sensitive cells and 15–30 hour intracellular half-life) enable administration via subcutaneous injection [Citation22,Citation23]. Reported toxicities mainly consist of transient bone marrow suppression predominantly affecting monocytes and neutrophil granulocytes [Citation14–17].
The first phase II trial of cladribine in adult LCH was evaluated in 1999 [Citation15]. Thirteen mostly pretreated patients received cladribine (with a median of three courses) at 0.1–0.14 mg/kg by two-hour intravenous infusion over five or seven consecutive days, every four weeks for a median of three courses. Of 12 valuable patients, seven and two achieved complete and partial responses, respectively. Overall response rate was 75%. In seven patients, grade 3 and 4 neutropenia occurred. Grau et al. [Citation16] analyzed a sample of nine, mostly adult patients with relapsed LCH. Most frequently, cladribine was administered at 0.1 mg/kg by two-hour or continuous intravenous infusion over five or seven consecutive days, every four weeks for a median of five courses. The overall response rate was 66% with two and four patients achieving complete and partial responses, respectively. One patient developed grade 4 neutropenia and in two cases grade 3 thrombocytopenia was observed. Retrospectively reviewed by Pardanani et al. [Citation17], all five, mostly pretreated patients with LCH attained an objective response (complete in three and partial in two cases). The treatment consisted of cladribine mostly at 0.1 mg/kg by two-hour or continuous intravenous infusion over seven consecutive days, for a median of four courses. Two patients developed grade 3 lymphopenia.
In this retrospective study we demonstrated excellent treatment results of cladribine in seven patients with newly diagnosed LCH or LCH without a history of systemic therapy. In six patients (86%) a durable complete remission has been achieved. One patient developed an aggressive disease early relapsing after two months. Patients with LCH resistant to cladribine may require intensive combination chemotherapy with additional agents or some of the novel experimental approaches. In Case 6, however, second-line treatment with CHOEP (cyclophosphamide, doxorubicin, vincristine, etoposide, prednisone) and high-dose BEAM (carmustine, etoposide, cytarabine, melphalan) chemotherapies resulted in a short-lasting remission and it was not until third-line treatment with lenalidomide regimen consolidated by allogeneic blood stem cell transplantation was administered that durable complete remission has been achieved. Based on several case-reports, other new promising drugs include tyrosine kinase inhibitors, imatinib [Citation24] and sorafenib [Citation25].
In our cohort, cladribine proved effective both as a single agent as well as in combination with alkylating cytostatic and corticoids. This finding is in agreement with observations in slowly progressing lymphoid malignancies, where combination of adenosine analogues with cyclophosphamide and dexamethasone yields higher response rates than cladribine or fludarabine alone [Citation26]. Additionally, Robak and co-workers successfully used cladribine and cyclophosphamide in a patient with aggressive multisystem LCH refractory to vincristine [Citation27]. Hence, such combination regimens should be considered in LCH non-responding or slowly responding to cladribine monotherapy, while keeping in mind the dose-dependent leukemogenicity of alkylators [Citation28].
Treatment-related toxicities, including transient bone marrow suppression, were mild and did not require any dosage adjustments or changes in the therapy plan. Grade 3 lymphopenia was observed in five patients (71%) and grade 3 neutropenia in one case (14%). It is generally accepted that diabetes insipidus is a life-long complication despite LCH remission, which is in accordance with our observations in two patients with pituitary infiltrations. Reports on reversibility of endocrinological functions are scarce [Citation29].
In conclusion, multisystem adult LCH is a rare disease with potentially life-threatening complications. The treatment outcomes to conventional therapeutic regimens have been inconsistent with the optimal therapeutic strategy yet to be defined. Cladribine given at a dose of 5 mg/m2 subcutaneously for five consecutive days represents an attractive alternative agent to vinblastine with favorable toxicity profile, considerable efficacy and convenient administration. Based on our observation, cladribine may become a leading drug in management of multisystem and aggressive multifocal adult LCH. Therefore, a randomized prospective clinical trial is a priority necessary for reaching definitive conclusions.
Notice of Correction
The version of this article published online ahead of print on 31 Aug 2012 contained an error on page 1, line 37 and on page 7, line 76.
The sentences on page 1 and 7, both which included the line “four patients (57%)” should have read “five patients (71%)”. The error has been corrected for this version.
Acknowledgements
This work was supported in part by research project of The Ministry of Education, Youth and Sports: MSM0021622434; IGA grants of The Ministry of Health: NT12130, NT12215; grant of The Czech Science Foundation GAP304/10/1395; MUNI/A/0784/2011 and European Regional Development Fund-Project FNUSA-ICRC (No.CZ.1.05/1.1.00/ 02.0123).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Egeler RM, D'Angio GJ. Langerhans cell histiocytosis. J Pediatr 1995;127:1–11.
- Weitzman S, Wayne AS, Arceci R, Lipton JM, Whitlock JA. Nucleoside analogues in the therapy of Langerhans cell histiocytosis: A survey of members of the histiocyte society and review of the literature. Med Pediatr Oncol 1999;33: 476–81.
- Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis – evolution and current approaches. Br J Cancer Suppl 1994;23:S41–6.
- Satter EK, High WA. Langerhans cell histiocytosis: A review of the current recommendations of the Histiocyte Society. Pediatr Dermatol 2008;25:291–5.
- Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol 1997;28:9–14.
- Aricò M, Girschikofsky M, Généreau T, Klersy C, McClain K, Grois N, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer 2003;39:2341–8.
- Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol 2006;76:363–8.
- McClain K, Allen C, Ebrahim S. Review of histiocytosis treatment and neurotoxicity in adult patients (abstract). Pediatr Blood Cancer 2009;53:696.
- Gulliford MC, Charlton J, Latinovic R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care 2006;29:2728–9.
- Histiocyte Society Evaluation and Treatment Guidelines. Available from: http://www.hematologie-amc.nl/odijk/bijlagennietinDBS/SocietyLCHTreatmentGuidelines.pdf.[cited 2012 April 3]
- Kassner E. Evaluation and treatment of chemotherapy extravasation injuries. J Pediatr Oncol Nurs 2000;17:135–48.
- Saven A, Figueroa ML, Piro LD, Rosenblatt JD. 2- Chlorodeoxyadenosine to treat refractory histiocytosis X. N Engl J Med 1993;329:734–5.
- Dimopoulos MA, Theodorakis M, Kostis E, Papadimitris C, Moulopoulos LA, Anastasiou-Nana M. Treatment of Langerhans cell histiocytosis with 2 chlorodeoxyadenosine. Leuk Lymphoma 1997;25:187–9.
- Weitzman S, Braier J, Donadieu J, Egeler RM, Grois N, Ladisch S, et al. 2’-Chlorodeoxyadenosine (2-CdA) as salvage therapy for Langerhans cell histiocytosis (LCH). Results of the LCH-S 98 protocol of the Histiocyte Society. Pediatr Blood Cancer 2009;53:1271–6.
- Saven A, Burian C. Cladribine activity in adult Langerhans-cell histiocytosis. Blood 1999;93:4125–30.
- Grau J, Ribera JM, Tormo M, Indiano JM, Vercher J, Sandoval V, et al. Results of treatment with 2-chlorodeoxyadenosine in refractory or relapsed Langerhans cell histiocytosis. Study of 9 patients. Med Clin (Barc) 2001;116:339–42. [Spanish].
- Pardanani A, Phyliky RL, Li CY, Tefferi A. 2-Chlorodeoxyadenosine therapy for disseminated Langerhans cell histiocytosis. Mayo Clin Proc 2003;78:301–6.
- Stine KC, Saylors RL, Williams LL, Becton DL. 2- Chlorodeoxyadenosine (2-CDA) for the treatment of refractory or recurrent Langerhans cell histiocytosis (LCH) in pediatric patients. Med Pediatr Oncol 1997;29:288–92.
- Szturz P, Adam Z, Rehák Z, Koukalová R, Slaisová R, Stehlíková O, et al. Lenalidomide proved effective in multisystem Langerhans cell histiocytosis. Acta Oncol 2012;51: 412–5.
- Carrera CJ, Terai C, Lotz M, Curd JG, Piro LD, Beutler E, et al. Potent toxicity of 2-chlorodeoxyadenosine toward human monocytes in vitro and in vivo. A novel approach to immunosuppressive therapy. J Clin Invest 1990;86:1480–8.
- Liliemark J. The clinical pharmacokinetics of cladribine. Clin Pharmacokinet 1997;32:120–31.
- Bryson HM, Sorkin EM. Cladribine. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in haematological malignancies. Drugs 1993;46:872–94.
- Liliemark J, Albertioni F, Hassan M, Juliusson G. On the bioavailability of oral and subcutaneous 2-chloro-2’- deoxyadenosine in humans: Alternative routes of administration. J Clin Oncol 1992;10:1514–8.
- Montella L, Insabato L, Palmieri G. Imatinib mesylate for cerebral Langerhans’-cell histiocytosis. N Engl J Med 2004;351:1034–5.
- Baumann M, Cerny T, Sommacal A, Koeberle D. Langerhans cell histiocytosis with central nervous system involvement-complete response to 2-chlorodeoxyadenosine after failure of tyrosine kinase inhibitor therapies with sorafenib and imatinib. Hematol Oncol 2012;30:101–4.
- Cheson BD. New prospects in the treatment of indolent lymphomas with purine analogues. Cancer J Sci Am 1998;4(Suppl 2):S27–36.
- Robak T, Kordek R, Robak E, Bartkowiak J, Biernat W, Liberski P, et al. Langerhans cell histiocytosis in a patient with systemic lupus erythematosus: A clonal disease responding to treatment with cladribine, and cyclophosphamide. Leuk Lymph 2002;43:2041–6.
- Felix CA. Chemotherapy-related second cancers. In: Neugut AI, Meadows AT, Robinson E, editors. Multiple primary cancers. Philadelphia (PA): Lippincott Williams and Wilkins; 1999.
- Ottaviano F, Finlay JL. Diabetes insipidus and Langerhans cell histiocytosis: A case report of reversibility with 2- chlorodeoxyadenosine. J Pediatr Hematol Oncol 2003;25: 575–7.