Abstract
Background. We investigated the radiographic and pathologic response rate of esophageal adenocarcinoma treated with neoadjuvant chemoradiation in patients taking metformin. Material and methods. Two hundred eighty-five patients with esophageal adenocarcinoma treated with concurrent chemoradiation (CRT) followed by esophagectomy from 1997 to 2012 were included in the study, including 29 diabetics taking metformin, 21 diabetics not taking metformin and 235 non-diabetics. Pre- and post-treatment positron emission tomography (PET) scans were available for 204 patients. Pathologic response was graded at the time of surgery. Response rates were compared using both the χ2 statistic as well as ANOVA with post-hoc LSD analysis. Multivariate logistic regression analysis was performed to control for predictors of pathologic complete response (CR) after CRT. Results. The overall rate of pathologic CR for the study population was 20%. The pathologic CR rate was higher in patients taking metformin (34.5%), compared to diabetic patients not taking metformin (4.8%, p = 0.01) and non-diabetic patients (19.6%, p = 0.05). Pathologic CR was related to metformin dose, with ≥1500 mg/d associated with a higher CR rate. No significant difference seen in pre-CRT maximum tumor SUV (p = 0.93), however post-CRT maximum SUV was significantly decreased in patients taking metformin (p = 0.05). On multivariate logistic regression, metformin use was independently associated with pathologic CR (p = 0.04). Metformin use was also associated with decreased in field loco-regional failure following radiation (p = 0.05). Conclusion. Metformin use is associated with a dose-dependent increased response to CRT in esophageal cancer and may be a sensitizer to this therapy.
Esophageal cancer is a deadly disease, with an estimated 406,800 deaths annually worldwide. The prognosis is particularly poor in patients with loco-regionally advanced disease at the time of presentation, with an estimated median survival of less than 30 months after chemoradiation and surgical resection based on SEER database analysis [Citation1]. Although there is some variability, one standard approach to treatment of these patients involves concurrent chemotherapy and radiation (CRT) prior to esophagectomy. Pathologic complete response (CR) rates following CRT vary from 10% to 40%, with CR being associated with improved survival [Citation2–4].
Recently, metformin has been investigated as an adjunct to standard chemotherapeutic regimens. Metformin use among diabetics has been linked to decreased rates of esophageal cancer [Citation5]. Pre-clinical data from several cancer cell types indicate that metformin has anti-tumor effects alone or in combination with either chemotherapy or radiation [Citation6–8]. This has been postulated to be via interaction with a number of signaling cascades. Metformin can inhibit insulin-like growth factor-1 (IGF-1) signaling by decreasing circulating insulin levels as well as the bioavailability of IGF-1 [Citation9]. Furthermore, metformin enhances the activation of the AMP kinase (AMPK) [Citation7]. Both of these pathways intersect at the level of the mammalian target of rapamycin (mTOR), which is also inhibited by metformin [Citation9]. However, data supporting the use of metformin during cancer therapy are not confined to pre-clinical studies. Metformin use is associated with higher CR rates in breast cancer patients treated with neoadjuvant chemotherapy as well as decreased rates of local failure following radiation in patients with head and neck cancer [Citation8,Citation10]. Thus, preclinical data as well as data from other disease sites hints at the possibility of metformin working in conjunction with standard therapy to improve response; however this question has not yet been addressed in esophageal cancer. Thus, the current study investigated the effect of metformin on both radiologic as well as pathologic CR after concurrent chemoradiation for locally advanced esophageal cancer. As a secondary outcome, the effect of metformin on locoregional control and survival outcomes was also examined.
Material and methods
Patient demographics
The charts of patients treated with concurrent chemoradiation followed by surgical resection for esophageal carcinoma from 1997 to 2012 were reviewed. Patients were required to be treated with curative intent and to have no visceral or bony metastatic disease at the time of surgery. A total of 285 patients met these criteria. Median follow-up for living patients was 21 months (0–162). The vast majority of patients were men (92%) who were either current or former smokers (81%) and had distal or gastroesophageal junction (GEJ) tumors (96.1%) (). Initial staging was performed in most patients via EGD and endoscopic ultrasound (EUS) as well as cross-sectional imaging [either chest computed tomography (CT) or positron emission tomography (PET)/CT]. A large proportion of patients had T3 disease (84%), with 63% having N1 disease and 35% having no positive lymph nodes at diagnosis. Fifty patients (18%) in this cohort were found to be diabetic, with either a pre-existing diagnosis of diabetes or blood glucose levels consistent with diabetes leading to a diagnosis at the time of presentation. Diabetic patients were then divided into two groups, those taking metformin alone or in addition to other anti-diabetic medications (29 patients), and those diabetics using other medications only (21 patients). This information was ascertained from the pharmacy record or from annotation in the patients chart at the time of radiation. No significant differences between these groups were seen in regards to gender, smoking status, tumor or nodal stage, or pathologic differentiation (). Further, no difference in insulin use was observed between the metformin group and the remaining diabetics (p = 0.17). Of note, patients with diabetes trended toward a poorer pre-treatment performance status, however this was not statistically significant (p = 0.008).
Table I. Patient demographics and treatment characteristics.
Table II. Treatment characteristics of the patient population.
Of the 285 patients in the current study, 204 (72%) had pre and post-treatment PET scans available for review. Pre-treatment PET scans were obtained at the time of presentation and post- treatment PET scans were obtained within 1–2 months following chemoradiation. The maximum SUV values of the patient's tumor were determined on both scans.
Treatment
The standard approach to treatment in locally advanced esophageal cancer at our institution is neoadjuvant radiation to the tumor and draining lymphatics (45–50.4 Gy), in combination generally with 5-FU and/or platinum-based chemotherapy. A significant minority of these patients (34%) were treated with induction chemotherapy, with agents listed in . There were no significant differences between groups in regards to induction chemotherapy treatment (p = 0.24) or type of radiation administered (p = 0.99) ().
Generally within eight weeks of completion of chemoradiation, an en-bloc esophagectomy with regional lymph node dissection was performed. Pathologic response was given as a percentage of remaining viable tumor by the pathologist at the time of surgery, with a complete response denoting no identifiable viable tumor in both the primary site and dissected lymph nodes.
Statistics
Patient demographics and tumor characteristics were compared between groups using the χ2 statistic. Pathologic complete response rates, as well as pre- and post-treatment tumor SUV were compared between groups using both the χ2 statistic as well as ANOVA with post-hoc LSD analysis. A multivariate logistical regression model was generated to examine pathologic response while controlling for tumor and nodal stage, differentiation, LVSI, and treatment with induction chemotherapy. A multivariate linear regression model was generated to examine post-treatment maximum SUV while controlling for the same variables. Percent pathologic response and post-treatment SUV were compared by use of Pearson correlation. Overall survival (OS), disease free survival (DFS), time to distant metastatic disease (DM) and time to loco-regional recurrence (LRR) were calculated using the Kaplan-Meier statistic with log rank comparisons performed to determine significance. For the purposes of this study, LRR was defined as a recurrence of disease within the radiation treatment field. All statistics were performed using SPSS (v.17). All p-values are two-sided, with a p-value of 0.05 considered significant.
Results
Radiographic response
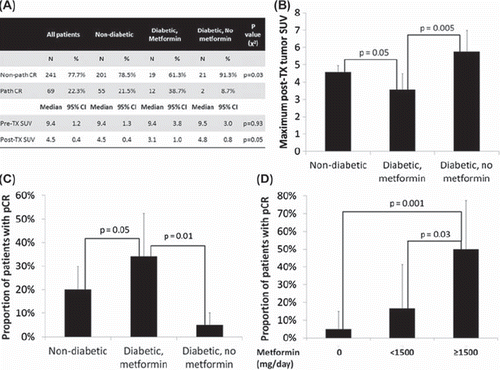
The median pre- and post-treatment maximum tumor SUV was 9.4 (95% CI 8.2–10.6) and 4.5 (95% CI 4.1–4.9), respectively, for the study population. On multivariate linear regression analysis, metformin use (p = 0.03) and LVSI (p = 0.002) were associated with post-treatment maximum SUV (). There was no significant difference between groups in pre-treatment SUV (). However, patients taking metformin at the time of chemoradiation had a significantly lower post- treatment SUV (mean 3.5) compared to non- diabetics (mean 4.6, p = 0.05) and diabetics not taking metformin (median 5.8, p = 0.005). Furthermore, post-treatment SUV was significantly correlated with percent pathologic response, with lower post-treatment SUV indicating less viable tumor (p = 0.00003).
Table III. Multivariate regression analysis of factors affecting pathologic CR rates.
Figure 1. Pathologic and radiolographic response rates between groups. (A) Pathologic CR rates and maximum tumor SUV between groups. (B & C) Bar graphs showing pathologic CR rate and post-treatment tumor SUV of each treatment group. (D) Pathologic response in diabetics stratified by daily dose of metformin (mg/day). p represents the p value of the comparison.
Pathologic complete response (CR)
The pathologic CR rate for the study population was 20%. On multivariate logistic regression analysis of patient and tumor characteristics only tumor stage (T3/4 vs. T1/2, p = 0.04), lymphovascular space invasion (LVSI) (p = 0.004) and metformin use (p = 0.04) were significantly associated with pathologic CR, with tumor differentiation (poor/unknown vs. well/moderate) trending towards significance (p = 0.07). Specifically, insulin use was not associated with pathologic CR rate (p = 0.3). In patients taking metformin at the time of CRT the pathologic CR rate was 35%, compared to 5% in diabetics not taking metformin and 20% in non-diabetics (). On post-hoc analysis, the pathologic CR rate was significantly greater in metformin users compared to either diabetics not taking metformin (p = 0.01) or non-diabetics (p = 0.05). Among all diabetics, a dose-response relationship was seen with metformin use, with higher pathologic CR rates in those patients taking 1500 mg/day or higher (16 patients), compared to those taking <1500 mg/day (12 patients, p = 0.03) or those taking no metformin (21 patients, p = 0.001) ().
Survival outcomes
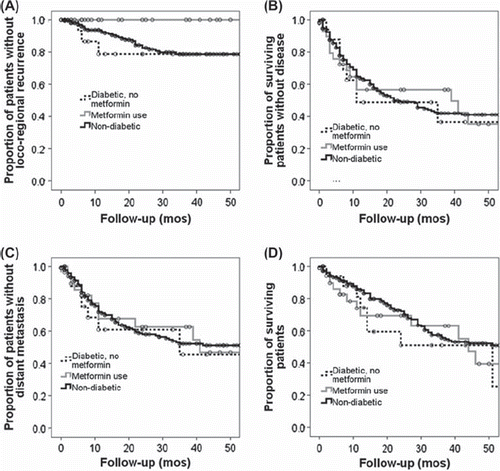
Median overall survival (OS) and disease free survival (DFS) for the study population were 51 and 24 months, respectively. No significant difference in median survival was seen between non-diabetics (56 months), diabetics taking metformin (44 months) and diabetics not taking metformin (51 months, p = 0.63). Median DFS was also not significantly different between groups (p = 0.92). However, median DFS was significantly improved among all patients with a pathologic CR compared to non-pathologic CR (64 months vs. 18 months, p = 0.01). Further, disease recurrence in the field of radiation was dramatically decreased in patients taking metformin (). Specifically, in patients taking metformin, the three year in field loco-regional control (LRC) rate was 100%, compared to 81% in the non-diabetic patients (p = 0.05) and 79% in diabetics not taking metformin (p = 0.03).
Figure 2. Outcomes for the study population. (A) Overall survival (OS), (B) Disease free survival (DFS), (C) Time to loco-regional recurrence (LRR) & (D) Time to distant metastatic disease (DM). LRR was significantly less in patients taking metformin compared to non-diabetics (p = 0.05) or diabetics not taking metformin (p = 0.04).
Discussion
The current study is the first to link metformin use to significantly improved radiographic and pathologic response to chemoradiation in esophageal cancer. This effect is present even after accounting for multiple variables known to affect response to CRT and argues for potentiation of either or both chemotherapy and radiation in this disease. This is similar to previous data indicating that metformin may improve response to chemotherapy [Citation10,Citation11] and radiation [Citation8] in other disease sites. Further, we found a significant dose-dependent effect of metformin on response, with doses of greater than 1500 mg/day, associated with improved pathologic CR.
The anti-tumor effect of metformin is not completely understood. One possibility is the addition of metformin can lead to decreased endogenous insulin levels. Insulin is mitogenic in a variety of cancer cell types, partially via activation of the insulin-like growth factor receptor-1 (IGF-1) in addition to the insulin receptor (IR) proper. Furthermore, obesity, and in particular the metabolic syndrome, have been associated with the development of Barrett's esophagus and progression to esophageal adenocarcinoma [Citation12,Citation13]. It is speculated that by downregulating this hyper-insulemic inflammatory state, metformin may improve response to therapy. However, in the current study, pathologic CR rates were higher in diabetics taking metformin than in the non-diabetic group, which is similar to the findings of Jiralerspong and colleagues, examining the effects of metformin in breast cancer [Citation10]. Although insulin levels in the non-diabetic population were not available for this study, presumably they were not grossly elevated, arguing against this hypothesis.
A secondary or complimentary explanation of the anti-tumor effect of metformin involves the AMP kinase (AMPK). Among other functions, this kinase is a critical energy sensor in the cell and is activated by a variety of cellular stressors including glucose deprivation and hypoxia. Furthermore, AMPK activation inhibits the function of the mammalian target of rapamycin (mTOR) signaling cascade which has been found to be activated in a variety of human tumors, including esophageal cancer [Citation14,Citation15]. Metformin has been shown to be a potent activator of AMPK in preclinical models which appears to be important to its activity as a monotherapy [Citation16,Citation17]. Furthermore, AMPK activation by chemotherapeutics as well as radiation has been shown to be potentiated in the presence of metformin leading to decreased mTOR signaling [Citation7,Citation8]. However, metformin can also exert inhibitory effects on mTOR signaling as well as anti-proliferative effects in an AMPK-independent manner [Citation18]. Thus, the precise interaction between metformin, AMPK and mTOR signaling is an ongoing field of study.
However, regardless of mechanism, metformin appears to have significant pre-clinical as well as clinical anti-tumor activity, but this may not be true for all tumors. The anti-tumor effects of metformin alone or in combination with cytotoxic therapy may be limited primarily to tumors with mutations in p53 [Citation6,Citation8,Citation19]. This phenomenon has been linked to direct effects on the mitochondria and, particularly upon the production of reactive oxygen species (ROS) [Citation6,Citation8,Citation19–21]. We have shown previously metformin potentiates an ROS dependent radiation-induced cell arrest, but only in the context of mutant p53 [Citation19,Citation22]. Furthermore, metformin alone or in combination with radiation, has been found to alter glucose metabolism, a process which can be impaired in mutant p53 cells [Citation6,Citation20,Citation22,Citation23]. As p53 mutations are found in at least 50% of all esophageal carcinomas and alterations in p53 signaling have been linked to poor response following local therapy, metformin may offer a novel method of targeting these resistant tumors.
In the current study a significantly decreased post-treatment SUV was observed in patients taking metformin compared to other groups. The effect of metformin on FDG uptake is unclear, with several studies showing increased or decreased FDG uptake after metformin treatment depending upon the model [Citation23–25]. This could be due to the dual action of metformin to both enhance AMPK-mediated signaling, which would increase glucose uptake, as well as decrease insulin secretion, with a concomitant decrease in glucose uptake. This phenomenon has led to speculation that FDG-PET would be a poor surrogate endpoint for response in trials examining metformin use. However, in the current study, we found that post-treatment SUV correlated with pathologic CR rates, thus providing some supporting data for the use of FDG-PET at least in the context of combined therapy.
The current study is limited by several factors, most notably its retrospective nature as well as the comparatively small numbers of patients in the diabetic groups. However, the groups are well balanced in regards to most known factors predicting pathologic response to local therapy in esophageal carcinoma. In the absence of a prospective clinical trial, the current data provides at least hypothesis generating data for the use of metformin in the neoadjuvant setting. Furthermore, although dramatic differences in response rates were seen, this did not translate to differences in OS or DFS. Unfortunately, although CR was associated with DFS in the current study (p = 0.01), this did not translate to a survival benefit in the patients taking metformin. This could be due to the low numbers of patients in the metformin arm masking a benefit in survival outcomes as well as the competing risk of underlying diabetes in this population. Interestingly, time to in field loco-regional recurrence in patients taking metformin at the time of CRT, was increased compared to other groups. Although most patients ultimately succumb to their disease due to distant failure, this pattern of metformin effect is noteworthy, in that it provides further data for synergy between metformin and the radiation component of CRT therapy in this disease.
In summary, the current study offers interesting data implicating metformin use in improved response to concurrent chemoradiation for esophageal carcinoma. Although, the current study provides limited data, we believe that this data, in combination with promising preclinical data as well as retrospective data from other disease sites warrants further prospective evaluation of metformin for the treatment of this devastating malignancy.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Kishi K, Doki Y, Miyata H, Yano M, Yasuda T, Monden M. Prediction of the response to chemoradiation and prognosis in oesophageal squamous cancer. Br J Surg 2002;89:597–603.
- Fields RC, Strong VE, Gönen M, Goodman KA, Rizk NP, Kelsen DP, et al. Recurrence and survival after pathologic complete response to preoperative therapy followed by surgery for gastric or gastrooesophageal adenocarcinoma. Br J Cancer 2011;104:1840–7.
- Meredith KL, Weber JM, Turaga KK, Siegel EM, McLoughlin J, Hoffe S, et al. Pathologic response after neoadjuvant therapy is the major determinant of survival in patients with esophageal cancer. Ann Surg Oncol 2010;17:1159–67.
- Merkow RP, Bilimoria KY, McCarter MD, Chow WB, Ko CY, Bentrem DJ. Use of multimodality neoadjuvant therapy for esophageal cancer in the United States: assessment of 987 hospitals. Ann Surg Oncol 2012;19:357–64.
- Lee M-S, Hsu C-C, Wahlqvist ML, Tsai H-N, Chang Y-H, Huang Y-C. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: A representative population prospective cohort study of 800,000 individuals. BMC Cancer 2011;11:20.
- Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 2007;67:6745–52.
- Rocha GZ, Dias MM, Ropelle ER, Osório-Costa F, Rossato FA, Vercesi AE, et al. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin Cancer Res 2011;17:3993–4005.
- Sanli T, Rashid A, Liu C, Harding S, Bristow RG, Cutz J-C, et al. Ionizing radiation activates AMP-Activated Kinase (AMPK): A target for radiosensitization of human cancer cells. Int J Radiat Oncol Biol Phys 2010;78:221–9.
- Aljada A, Mousa SA. Metformin and neoplasia: Implications and indications. Pharmacol Ther 2012;133:108–15.
- Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol 2009;27:3297–302.
- Romero IL, McCormick A, McEwen KA, Park S, Karrison T, Yamada SD, et al. Relationship of type II diabetes and metformin use to ovarian cancer progression, survival, and chemosensitivity. Obstet Gynecol 2012;119:61–7.
- Lin S-W, Freedman ND, Hollenbeck AR, Schatzkin A, Abnet CC. Prospective study of self-reported diabetes and risk of upper gastrointestinal cancers. Cancer Epidemiol Biomarkers Prev 2011;20:954–61.
- Ryan AM, Duong M, Healy L, Ryan SA, Parekh N, Reynolds JV, et al. Obesity, metabolic syndrome and esophageal adenocarcinoma: Epidemiology, etiology and new targets. Cancer Epidemiol 2011;35:309–19.
- Hirashima K, Baba Y, Watanabe M, Karashima R, Sato N, Imamura Y, et al. Phosphorylated mTOR expression is associated with poor prognosis for patients with esophageal squamous cell carcinoma. Ann Surg Oncol 2010;17:2486–93.
- Hou G, Xue L, Lu Z, Fan T, Tian F, Xue Y. An activated mTOR/p70S6K signaling pathway in esophageal squamous cell carcinoma cell lines and inhibition of the pathway by rapamycin and siRNA against mTOR. Cancer Lett 2007;253:236–48.
- Ben Sahra I, Tanti J-F, Bost F. The combination of metformin and 2 deoxyglucose inhibits autophagy and induces AMPK-dependent apoptosis in prostate cancer cells. Autophagy 2010;6:670–1.
- Zhuang Y, Miskimins WK. Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMK, downregulation of cyclin D1, and requires p27Kip1 or p21Cip1. J Mol Signal 2008;3:18.
- Ben Sahra I, Regazzetti C, Robert G, Laurent K, Le Marchand-Brustel Y, Auberger P, et al. Metformin, independent of AMPK, induces mTOR inhibition and cell-cycle arrest through REDD1. Cancer Res 2011;71:4366–72.
- Skinner HD, Sandulache VC, Ow TJ, Meyn RE, Yordy JS, Beadle BM, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res 2012;18:290–300.
- Sandulache VC, Ow TJ, Pickering CR, Frederick MJ, Zhou G, Fokt I, et al. Glucose, not glutamine, is the dominant energy source required for proliferation and survival of head and neck squamous carcinoma cells. Cancer 2011;117:2926–38.
- Martinez-Outschoorn UE, Goldberg A, Lin Z, Ko Y-H, Flomenberg N, Wang C, et al. Anti-estrogen resistance in breast cancer is induced by the tumor microenvironment and can be overcome by inhibiting mitochondrial function in epithelial cancer cells. Cancer Biol Ther 2011;12:924–38.
- Sandulache VC, Skinner HD, Ow TJ, Zhang A, Xia X, Luchak JM, et al. Individualizing antimetabolic treatment strategies for head and neck squamous cell carcinoma based on TP53 mutational status. Cancer 2012;118:711–21.
- Mashhedi H, Blouin M-J, Zakikhani M, David S, Zhao Y, Bazile M, et al. Metformin abolishes increased tumor (18)F-2-fluoro-2-deoxy-D-glucose uptake associated with a high energy diet. Cell Cycle 2011;10:2770–8.
- Gontier E, Fourme E, Wartski M, Blondet C, Bonardel G, Le Stanc E, et al. High and typical 18F-FDG bowel uptake in patients treated with metformin. Eur J Nucl Med Mol Imaging 2008;35:95–9.
- Ozülker T, Ozülker F, Mert M, Ozpaçaci T. Clearance of the high intestinal (18)F-FDG uptake associated with metformin after stopping the drug. Eur J Nucl Med Mol Imaging 2010;37:1011–7.