Abstract
Objective. To report two year clinical outcomes of image guided radiation therapy (IGRT) to the vaginal cuff and pelvic lymph nodes in a series of high-risk endometrial cancer patients. Methods. Twenty-six consecutive high-risk endometrial cancer patients requiring adjuvant radiation to the vaginal cuff and regional lymph nodes were treated with vaginal cuff fiducial-based IGRT. Seventeen (65%) received sequential chemotherapy, most commonly with a sandwich technique. Brachytherapy followed external radiation in 11 patients to a median dose of 18 Gy in 3 fractions. The median external beam dose delivered was 47.5 Gy in 25 fractions. Results. All 656 fractions were successfully imaged and treated. The median overall translational shift required for correction was 9.1 mm (standard deviation, 5.2 mm) relative to clinical set-up with skin tattoos. Shifts of 1 cm, 1.5 cm, and 2 cm or greater were performed in 43%, 14%, and 4% of patients, respectively. Acute grade 2 gastrointestinal (GI) toxicity occurred in eight patients (30%) and grade 3 toxicity occurred in one. At two years, there have been no local or regional failures and actuarial overall survival is 95%. Conclusion. Daily image guidance for high-risk endometrial cancer results in a low incidence of acute GI/genitourinary (GU) toxicity with uncompromised tumor control at two years. Vaginal cuff translations can be substantial and may possibly result in underdosing if not properly considered.
Adjuvant radiation after hysterectomy for endometrial cancer plays a well established role both in intermediate- [Citation1–4] and high-risk patients [Citation5–7]. Historically, a four-field technique has been used to encompass the pelvic lymph nodes and vaginal cuff. This technique exposes a substantial portion of small bowel, bladder, and rectum to 45–50 Gy and leads to considerable acute and late morbidity. Numerous dosimetric studies over the past decade have investigated intensity modulated radiation therapy (IMRT) as an alternative to the four-field technique, with most showing considerable sparing of normal tissues and improved target coverage [Citation8–14]. Early clinical reports investigating IMRT for endometrial cancer have supported its use [Citation15–18].
Precise delivery of radiation is only useful when it is accompanied by accurate target alignment and reproducibility. As with other sites, the targets involved in endometrial cancer are prone to internal motion and set-up variability, making set-up based on skin markings or bony anatomy less than ideal. Early investigations of vaginal cuff motion suggested sizable magnitudes with relatively unpredictable directionality [Citation19]. One report from Washington University suggested average vaginal cuff movements of 16.2 mm per day with maximum movements exceeding 34 mm [Citation20]. Given the propensity for vaginal cuff motion, we investigated image guided radiation therapy (IGRT) targeting the vaginal cuff as a method to improve accuracy of IMRT delivery in high-risk post-operative endometrial cancer patients.
Material and methods
Twenty-six consecutive women with high-risk endometrial cancer were studied under an Investigative Review Board approved retrospective study. All patients underwent hysterectomy, bilateral salpingo-oopherectomy, pelvic lymphadenectomy, para-aortic lymph node sampling as indicated by stage, and evaluation of pelvic washings. All surgical cases were performed by a single gynecologic oncologist (D. P.).
Patient characteristics are reported in . Staging is reported per the American Joint Committee on Cancer (AJCC) 7th edition. All patients were considered to be at sufficient risk to warrant vaginal cuff and pelvic lymph node radiation in the adjuvant setting. The median patient age was 62 years old. The median tumor size was 4 cm and the median myometrial invasion was 65%. Fifteen of 26 patients had high grade disease and 16 patients had angio-lymphatic invasion. Thirteen patients had positive lymph nodes, four of whom had positive para-aortic lymph nodes.
Table I. Patient characteristics.
Use of fiducials
Twenty-one women underwent placement of one (n=4) or two (n=17) fiducial markers in the clinic just prior to simulation; four patients were imaged with daily cone beam computed tomography (CBCT) scans, and one with a pelvic bone metastasis underwent a combination of CBCT and orthogonal imaging. Placement of one fiducial was abandoned early in the experience as inadequate. The decision to use two fiducials instead of the three that would be required to fully assess six degrees of freedom was based on two factors. First, the vaginal cuff target is essentially a linear band of fibrotic scar corresponding to the vaginal anastamosis line; thus, attempts to place a third fiducial more proximally would run the risk of bowl perforation and placement more distally would involve placement in the lateral vaginal wall away from the actual target. Second, we felt that independent movement between two fiducials would be negligible given their close proximity. Under this assumption, a rigid body system is created that can be adequately characterized by fewer fiducials. Since conventional treatment couches can only correct for x, y, and z translations and couch angle corrections along the axis of the table, we elected to compensate for any possible rotation or yaw differences via the four degrees of freedom available to the therapist at the time of treatment.
Simulation and fiducial placement
CT simulation was performed with intravenous contrast when not contraindicated clinically. In each case, patients were positioned in stirrups on the CT table. Betadine swabs were used to prepare the vaginal cuff. Preloaded, 18-gauge needles (containing 0.75×5 mm gold coiled fiducials) were used to place the fiducials in the upper vaginal fornicies just beneath the mucosa. Placement was well tolerated in each case with minimal discomfort or bleeding. Local anesthesia was not used. CT simulation was performed immediately after placement of fiducials.
Treatment planning details
Tumor volumes were contoured following the RTOG post-operative GYN atlas available online [Citation21]. IMRT plans were then generated; the first 10 patients were planned using a step-and-shoot technique with seven to nine beams and the last 16 patients were planned using volumetric modulated arc therapy (VMAT) with the Pinnacle planning platform for delivery on the Varian Trilogy®.
Two clinical target volumes (CTV) were generated for each case: a vaginal cuff CTV to include the upper one third of the vagina and surrounding tissue at risk and a pelvic lymph node CTV surrounding the iliac vessels to the L4/5 interspace, typically. The four patients with stage IIIC2 disease were treated with extended fields to include the para-aortic nodes, typically to the T12-L1 interspace or as dictated by pre-operative imaging. Planning target volume (PTV) expansions ranged from 7–10 mm for both CTVs. A median dose of 47.5 Gy in 25 fractions was prescribed to the vaginal cuff PTV and the nodal PTV was treated to 45 Gy in 25 fractions.
Bladder, rectum, bowel, and femoral necks were contoured as critical structures for each case. Small bowel was constrained to a max dose of 45 Gy. In instances where a loop of bowel overlapped the vaginal cuff PTV, coverage was prioritized as long as the maximum bowel dose could be held below 50 Gy. Typically, two symmetric VMAT arcs were required to achieve adequate coverage and conformality.
IMRT plans were subsequently reviewed and the bowel V40 (percent receiving 40 Gy), bladder V45, rectal V40, and femoral neck V35 were recorded for correlation with acute toxicity outcomes. Each week, acute gastrointestinal (GI) and genitourinary (GU) toxicity scores were prospectively assigned using the Common Toxicity Criteria (CTC v.3).
Image guidance procedure
The fiducial markers were identified on the planning CT scan and outlined as contours. Digitally reconstructed radiographs were produced with the fiducial contours as a wireframe structure. Patients imaged with kilovoltage orthogonal x-rays underwent manual matching of the fiducials with these contours in order to determine translational offsets. Couch correction angles were determined as well. X-ray based shifts were calculated relative to clinical set-up with skin tattoos, as this was our standard procedure prior to implementing an IGRT program. Translations were recorded in the x, y, and z directions and an overall translation vector was also recorded which represents the square root of the sum of the squares of each separate translation. All shifts were performed regardless of magnitude; there was no threshold for correction. Even though alignment was based on vaginal cuff position, CBCT scans were performed once a week on those with fiducials to verify acceptable alignment of nodal CTVs.
High dose rate brachytherapy was delivered at the conclusion of external beam radiation for 11 patients. Eight received 18 Gy in 3 fractions and three received 12 Gy in 2 fractions, each prescribed to the vaginal surface and delivered over approximately two weeks with at least two days between procedures.
Chemotherapy was delivered to 17 of 26 patients (65%). All nine patients not receiving chemotherapy had either stage Ib, grade 3 disease or stage II disease. TAP chemotherapy (Taxol, Adriamycin, Cisplatin) was used in five cases, Carboplatin/Taxol was used in nine, and a combination of these regimens was used in three cases. The median number of chemotherapy cycles was six with the most common sequence involving a sandwich technique delivering three cycles before and three cycles after radiation.
Statistics
Possible explanatory variables for acute GI toxicity grade 2 were investigated using the Wilcoxon rank sum and Fisher's exact test for continuous and nominal variables, respectively. These variables included: hypertension, previous abdominal surgery, stage (I–II vs. III–IV), extended field technique, brachytherapy use, chemotherapy use, rectal V40, bowel V40, age, weight, and number of nodes dissected. Acute GU toxicity analysis was similar, with the only exception being replacement of rectal and bowel dosimetry parameters with the bladder V45. Two-sided p-values were used and considered significant at the 0.05 level. Multivariate analysis was not felt to be a valid endeavor due to the limited number of toxicity events. Median follow-up after hysterectomy is 2.1 years. Median follow-up from radiation is 1.9 years (range 0.9–3.6 years). One patient was lost to follow-up after 1.65 years.
Results
A total of 656 fractions were successfully imaged and treated with IGRT. Of the 17 patients having two fiducials placed, two patients lost one of the fiducials between simulation and the start of treatment; in both cases, the remaining fiducial was successfully tracked for the entire course (ignoring the effects of rotation).
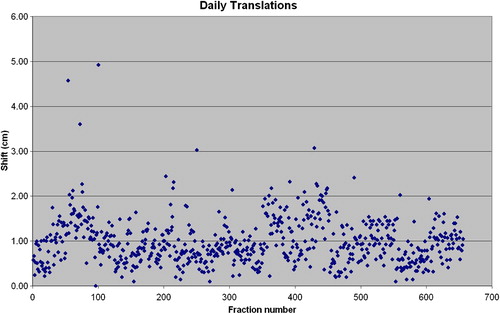
The median translational shift required for correction was 9.1 mm (standard deviation, 5.2 mm) relative to clinical set-up with skin tattoos. Average patient shifts in the superior/inferior, lateral, and anterior/posterior directions were 4.1 mm, 5.5 mm, and 4.1 mm, respectively. Composite shifts of 1 cm, 1.5 cm, and 2 cm or greater were performed in 43%, 14%, and 4% of patients, respectively. shows the translation performed for each fraction.
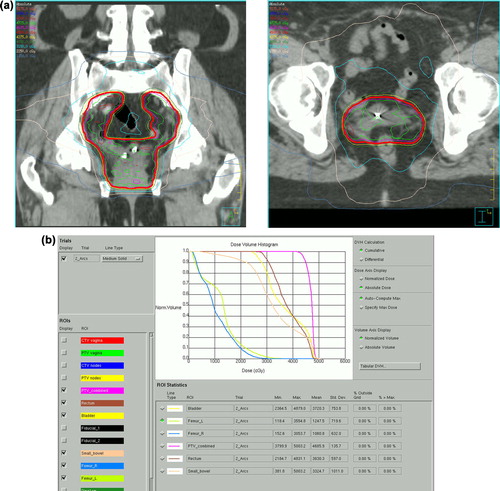
The median rectal V40 was 43% (range 4–94). The median bladder V45 was 42% (range 18–94), which is slightly higher than the goal of 35% sought by the RTOG 0921 clinical trial, typically due to incomplete bladder filling. The median bowel V40 was 24% (range 0–67). The individual femoral neck receiving the higher dose was recorded as the femoral neck V35; the median value was 4% (range 0–20). represents a sample dose-volume histogram and corresponding dose distribution in the vaginal cuff region.
Figure 2. a) Sample coronal dose distribution at the level of the vaginal cuff fiducials. b) Dose-volume histogram for sample patient.
Acute grade 2 gastrointestinal (GI) toxicity occurred in eight patients and grade 3 toxicity occurred in one. In all cases grade 2 toxicity manifest as diarrhea between four and six times per day, associated with vague abdominal cramping and discomfort. The single case of grade 3 toxicity was a patient with more than seven loose stools per day over baseline and this was subsequently controlled with only anti-diarrheal medicine. On univariate analysis (), the only significant predictor of grade 2 GI toxicity was the rectal V40. (63.3% vs. 35.9%; p=0.007) Acute grade 2 genitourinary (GU) toxicity (cystitis) was observed in three patients; no factors analyzed were predictive for acute GU toxicity.
Table II. Univariate analysis.
At two years of actuarial follow-up, no patient has experienced a local or regional failure. In addition to the two patients with metastatic disease at presentation, one additional patient developed a distant recurrence seven months after completing radiation and subsequently died. Two year overall survival (excluding the two patients with metastatic disease at presentation) is 95%.
There has been a single case of grade 3 late GI toxicity in which argon plasma coagulation (APC) laser treatment was delivered 13 months after completion of external radiation and brachytherapy for radiation proctitis. One 80-year-old patient (with hip V35 of 18%) developed avascular necrosis changes on magnetic resonance imaging (MRI) approximately 10 months after RT and subsequently underwent hip replacement.
Discussion
To our knowledge, this is the first report of IGRT ± chemotherapy for high-risk endometrial cancer patients with clinical outcome data. Our early clinical experience has been very favorable with low rates of GI and GU toxicity compared with standard four-field techniques and no local-regional recurrences [Citation15–18].
Image guidance targeting the vaginal cuff was felt to be an important consideration given the potential for underdosing targets and overdosing nearby critical structures like bladder and rectum. Previous reports on the magnitude of vaginal cuff motion have been inconsistent. An abstract presented at ASTRO in 2010 from Washington University studied 11 patients with megavoltage CT [Citation20]. Fusion was performed based upon bony anatomy and the position of the fiducial markers was compared with the planning CT scan. In this report, the average vaginal cuff movement as 16.2 mm with a standard deviation of 8.3 mm. Maximum movements of 34.5 mm were reported. The authors reported that the probability of vaginal cuff excursion outside of the clinical target volume is 62% if 1 cm margins are used without image guidance. Even with a 2 cm margin, there remains a 14% probability of vaginal cuff excursion and potential marginal miss.
A separate report in 2009 from Moffitt Cancer Center investigated 13 patients and reached a different conclusion [Citation22]. They report a 25% incidence of fiducial loss after placement which is slightly higher than we have seen. The average motion of the center of mass of three fiducials was calculated as 2.1 mm with a standard deviation of 2 mm (range 0–18 mm). They conclude that 95% of fiducial excursions could be encompassed with a 7 mm PTV expansion.
The Pittsburgh group has studied gynecologic IMRT extensively and reported their results of vaginal cuff target motion recently [Citation23]. They suggested a PTV margin of approximately 10 mm for those with body mass index (BMI) 30 and 7 mm for those with lower BMIs. The University of Pennsylvania similarly demonstrated utility of IGRT for those with high BMI [Citation24].
A group from the Netherlands recently published their experience with internal motion of the vagina using MRI before and weekly during radiation [Citation25]. They found the anterior-posterior excursions to be most significant with 95% coverage achievable with margins up to 2.3 cm in this dimension. When 1.0 cm and 1.5 cm uniform margins were investigated, incomplete coverage was noted 53% and 20% of the time, respectively.
The RTOG is currently undertaking a phase II study of IMRT for endometrial cancer patients (www.rtog.org). In this study, two treatment planning scans are required: one with a full bladder and one with an empty bladder. A vaginal cuff integrated target volume (ITV) is then produced from the union of contours on these datasets. A 7 mm PTV expansion is added to the ITV to account for set-up uncertainty. This approach exposes the entire range of possible locations of the vaginal cuff to radiation through the cycle of bladder filling and emptying. Our approach differs in that the vaginal cuff is specifically targeted each day based upon its location at the time, potentially limiting dose to adjacent normal structures even further.
One issue that arises with specifically targeting the vaginal cuff is possible misalignment of the pelvic nodes. This situation is similar to that encountered in prostate and anal IMRT. Until recently, most IMRT was performed without image guidance, which essentially ignores the problem of internal motion (both nodes and cuff). A possible solution is to align both the vaginal cuff and nodal volumes based on daily CBCT; however, this adds time and integral radiation dose to the patient. It also requires some degree of compromise between alignments of the two areas. Our solution was to align primarily to the vaginal cuff and then verify the clinical appropriateness of nodal target coverage with weekly CBCT. While these structures may move independently, we found that reasonable coverage of nodal volumes was maintained on the CBCT verification scans. It is possible that a vaginal cuff ITV approach may cover the nodal volumes more robustly; however, this is at the expense of treating the bladder and rectum to higher overall daily doses in an effort to cover all possible locations of the vaginal cuff. To be conservative, one could set a threshold translation beyond which a confirmatory CBCT is performed to assess nodal coverage, or simply increase the nodal PTV expansions beyond 7–10 mm. A more comprehensive analysis of nodal target mismatch is beyond the scope of this article but would be an interesting topic for further research. Ultimately, nodal recurrences have not been demonstrated in our very high-risk population of patients with a median follow-up of two years. Additional follow-up is required to confirm these results.
Retrospective analyses of toxicity are never as robust as a well performed prospective trial; however, our experience has been overall quite favorable in terms of patient tolerance. Our 30% incidence of grade 2 GI toxicity and 11% incidence of grade 2 GU toxicity compares favorably with other large series. Scotti et al. reported a 20% incidence of acute diarrhea (mostly grade 1 and 2) with conventional techniques in a population of early stage patients [Citation26]. Conversely, Bouchard et al. have published on aperture-based IMRT treatment of endometrial cancer and report an 87% incidence of acute grade 2 or higher GI toxicity [Citation18]. This was higher than their comparative population treated with conventional techniques and certainly higher than the rate observed in this study. Possible explanations for Bouchard's higher toxicity rate include lack of constraint on lower isodose lines to bowel and the presence of hotspots in the rectum. Careful attention to the rectal V40, lower dose isodose lines, and presence/location of hot spots is essential to minimizing risk in treatment planning. Given that a substantial portion of our population was advanced stage and had received chemotherapy prior to radiation, our acute toxicity rates seem very encouraging.
In terms of clinical outcomes, our early data compares favorably with preliminary results of RTOG 9708 which investigated chemo-radiation in a similar population of patients. Greven et al. reported 24 month pelvic and regional failure rates of 2% and 3%, respectively, and an overall two year survival of 90% [Citation27]. Toxicity rates in RTOG 9708 were higher with 27% experiencing grade 3 and 2% experiencing grade 4 acute toxicity. The higher toxicity rates in RTOG 9708 could relate to conventional radiation techniques, more robust prospective toxicity assessment, use of chemotherapy in all patients, or a combination of factors. More mature results from RTOG 9708 confirm excellent local-regional control at a median follow-up of 4.3 years [Citation28].
Strength of this study is the potential applicability of our findings to the community setting. All patients were treated by one of two radiation oncologists in a hospital-based community cancer center. Weaknesses of the study include limited patient numbers and the inherent biases of retrospective research. Our GU toxicity analysis could have been more robust had we insisted on uniform bladder filling at the time of simulation and treatment; however, consistency in this regard is often difficult to achieve. Finally, our follow-up is relatively short at two years; late recurrences and long-term effects of treatment are not adequately addressed as a result.
Conclusion
Daily image guidance for high-risk endometrial cancer is feasible and results in a low incidence of acute GI/GU toxicity with uncompromised local regional control. Vaginal cuff translations can be substantial and may possibly result in underdosing if not properly considered.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Scholten AN, van Putten WL, Beerman H, Smit VT, Koper PC, Lybeert ML, et al. Postoperative radiotherapy for stage 1 endometrial carcinoma: Long-term outcome of the randomized PORTEC trial with central pathologic review. Int J Radiat Oncol Biol Phys 2005;63:834–8.
- Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 2004;92:744–51.
- Aalders J, Abeler V, Kolstad P, Onsrud M. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients. Obstet Gynecol 1980;56:419–27.
- ASTEC/EN.5 Study Group, Blake P, Swart AM, Orton J, Kitchener H, Whelan T, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): Pooled trial results, systematic review, and meta-analysis. Lancet 2009;373:125–36.
- Randall ME, Filiaci VL, Muss H, Spirtos NM, Mannel RS, Fowler J, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol 2006;24:36–44.
- Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, et al. Adjuvant chemotherapy vs. radiotherapy in high-risk endometrial carcinoma: Results of a randomized trial. Br J Cancer 2006;96:266–71.
- Klopp AH, Jhingran A, Ramondetta L, Lu K, Gershenson DM, Eifel PJ. Node-positive adenocarcinoma of the endometrium: Outcome and patterns of recurrence with and without external beam irradiation. Gynecol Oncol 2009; 115:6–11.
- Roeske JC, Lujan A, Rotmensch J, Waggoner SE, Yamada D, Mundt AJ. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys 2000;48:1613–21.
- Igdem S, Ercan T, Alco G, Zengin F, Ozgules R, Geceer G, et al. Dosimetric comparison of intensity modulated pelvic radiotherapy with 3D conformal radiotherapy in patients with gynecologic malignancies. Eur J Gynaecol Oncol 2009;30:547–51.
- Yang R, Xu S, Jiang W, Wang J, Xie C. Dosimetric comparison of postoperative whole pelvic radiotherapy for endometrial cancer using three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and helical tomotherapy. Acta Oncol 2010;49:230–6.
- Heron DE, Gerszten K, Selvaraj RN, King GC, Sonnik D, Gallion H, et al. Conventional 3D conformal versus intensity-modulated radiotherapy for the adjuvant treatment of gynecologic malignancies: A comparative dosimetric study of dose-volume histograms small star, filled. Gynecol Oncol 2003;91:39–45.
- Chan P, Yeo I, Perkins G, Fyles A, Milosevic M. Dosimetric comparison of intensity-modulated, conformal, and four-field pelvic radiotherapy boost plans for gynecologic cancer: A retrospective planning study. Radiat Oncol 2006;1:13.
- Georg P, Georg D, Hillbrand M, Kirisits C, Potter R. Factors influencing bowel sparing in intensity modulated whole pelvic radiotherapy for gynaecological malignancies. Radiother Oncol 2006;80:19–26.
- Lian J, Mackenzie M, Joseph K, Pervez N, Dundas G, Urtasun R, et al. Assessment of extended-field radiotherapy for stage IIIC endometrial cancer using three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, and helical tomotherapy. Int J Radiat Oncol Biol Phys 2008;70:935–43.
- Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys 2003;56:1354–60.
- Salama JK, Mundt AJ, Roeske J, Mehta N. Preliminary outcome and toxicity report of extended-field, intensity-modulated radiation therapy for gynecologic malignancies. Int J Radiat Oncol Biol Phys 2006;65:1170–6.
- Tierney RM, Powell MA, Mutch DG, Gibb RK, Rader JS, Grigsby PW. Acute toxicity of postoperative IMRT and chemotherapy for endometrial cancer. Radiat Med 2007;25:439–45.
- Bouchard M, Nadeau S, Gingras L, Raymond PE, Beaulieu F, Beaulieu L, et al. Clinical outcome of adjuvant treatment of endometrial cancer using aperture-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008;71:1343–50.
- Kim CR, Eaton BA, Stevens DR Jr.Localization of the apex of the vagina: Implications for radiation therapy planning. Radiology 1999;212:155–8.
- Ma DJ, Michaletz-Lorenz M, Goddu SM, Grigsby PW. Magnitude of interfractional vaginal cuff movement: Implications for external irradiation. Int J Radiat Oncol Biol Phys 2012;82:1439–44.
- Radiation Therapy Oncology Group website. Available from: http//www.rtog.org/CoreLab/ContouringAtlases/GYN
- Harris EE, Latifi K, Rusthoven C, Javedan K, Forster K. Assessment of organ motion in postoperative endometrial and cervical cancer patients treated with intensity-modulated radiation therapy. Int J Radiat Oncol Biol Phys 2011;81:e645–50.
- Kim H, Beriwal S, Huq MS, Kannan N, Shukla G, Houser C. Evaluation of set-up uncertainties with daily kilovoltage image guidance in external beam radiation therapy for gynecological cancers. Clin Oncol (R Coll Radiol) 2012;24:e39–45.
- Lin L, Hertan L, Rengan R, Teo BK. Effect of body mass index on magnitude of setup errors in patients treated with adjuvant radiotherapy for endometrial cancer with daily image guidance. Int J Radiat Oncol Biol Phys 2012;83:670–5.
- Jurgenliemk-Schulz IM, Toet-Bosma MZ, de Kort GA, Schreuder HW, Roesink JM, Tersteeg RJ, et al. Internal motion of the vagina after hysterectomy for gynaecological cancer. Radiother Oncol 2011;98:244–8.
- Scotti V, Borghesi S, Meattini I, Saieva C, Rossi F, Petrucci A, et al. Postoperative radiotherapy in stage I/II endometrial cancer. Retrospective analysis of 883 patients treated at the University of Florence. Int J Gynecol Cancer 2010;20:1540–8.
- Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Preliminary analysis of RTOG9708: Adjuvant postoperative radiotherapy combined with cisplatin/paclitaxel chemotherapy after surgery for patients with high-risk endometrial cancer.Int J Radiat Oncol Biol Phys2004;59:168–73.
- Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG9708: Adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer.Gynecol Oncol2006;103:155–9.