Abstract
Background. Standard treatment is surgery with stage dependent postoperative radio(chemo)therapy, however, for organ preservation preoperative radio(chemo)therapy is used as an individual approach. The present analysis was performed to access outcome and toxicity of radiotherapeutical treatment of squamous cell carcinoma of the tongue. Patients and methods. Sixty-six patients (median age 55 years) with cancer of the mobile tongue (n30) or tongue margins (n36) treated between 1982 and 2006 were retrospectively analyzed. Treatment consisted of definitive- (n13, median dose 66 Gy), adjuvant- (n31, median dose 60 Gy) or neoadjuvant radiotherapy (n22, median dose 40 Gy) and chemotherapy (n34) or immunotherapy (n1). Results. After a median follow-up of 29 months the three- and five-year overall survival (OS) rates were 59% and 46%, respectively. The median OS was 54 months. Forty-two patients achieved complete remission whereas 14 patients showed partial remission. The one- and two-year loco-regional progression-free survival (LRPFS) rates were 76% and 58%, respectively. The median LRPFS time was 36 months. In χ2-test, T-stage showed a trend towards impact on local recurrence (Pearson, p0.082). In multivariate analysis, alcohol consumption (p0.003) and gender (p0.031) were prognostic. Grade III/IV acute toxicity was seen in 52% of patients. None of the locally controlled patients reported grade IV or higher late toxicity. Conclusion. No statistically significant differences between treatment modalities were found, but one should keep in mind that organ preservation plays a major role for quality of life. None of the locally controlled patients reported grade IV or higher late toxicity. However, tumor recurrence is common, especially in advanced tumor stage. Interdisciplinary concepts, further increasing the chance of tumor control are warranted.
Oral carcinomas in, e.g. Nova Scotia accounted for 2% of all cancer cases as reported by Howell et al. Twenty percent of these oral carcinomas were carcinomas of the tongue. The rate increased by 23% and the age-standardized incidence rate increased by 10% over a 15 year period [Citation1]. The estimated number of new tongue cancer cases in 2009 in the US was 10 530, the estimated mortality 1910 [Citation2]. Risk factors for oral and oropharyngeal cancers published in 2005 by Rosenquist were smoking, alcohol consumption, poor oral hygiene, inadequate dental status and malfunctioning complete dentures [Citation3]. Furthermore, subtypes of the human papilloma virus (HPV) were associated with squamous cell carcinoma of the head and neck region as well as an increased relative risk for recurrence and secondary primary tumors [Citation3–5]. Surgery is standard treatment of advanced tongue cancer, e.g. as de-escalated surgery in early tumor stages as reported by Barry and colleagues [Citation6], but still, loss of organ function may be a major concern. However, optimal strategies are discussed and patients commonly receive individual treatment. Therefore, management regimens include not only surgery but also brachytherapy and external beam irradiation [Citation7–13]. Other approaches like neoadjuvant taxan-based chemotherapy may also play a role in the future [Citation14]. The aim of this retrospective single institution analysis was to access outcome and toxicity of treatment in carcinomas of the oral tongue and to compare different approaches of surgery combined with radiotherapy or radiotherapy alone regarding treatment success.
Patients and methods
Patient characteristics
Between 1980 and 2006, 66 patients with a median age of 55 years (range, 29–87 years; 29 female, 37 male) were treated for squamous cell carcinoma of the mobile tongue (n30) or tongue margins (n36) at the Department of Radiation Oncology, University Hospital of Heidelberg. Six additional patients were excluded from this analysis due to a very short follow-up time (/3 months). The treatment consisted of radiotherapy in combination with platinum-based chemotherapy (n34) or immunotherapy (n1) and surgery [tumor resection (n53), neck dissection (n35), suprahyoidal dissection (n32)]. The disease stages were found in . Furthermore, a subgroup of 16 patients had T1-2 N0 disease. Retrospectively, immunohistologic markers were not adequately accessible. Further patient characteristics were listed in .
Table I. Patient characteristics.
Radiotherapy
Megavoltage equipment (6 MeV) was used. Radiotherapy was performed by simulator-based, 3D-computerized multifield treatment planning techniques and in recent years, inverse planned, intensity-modulated radiation therapy (IMRT). Patient fixation was done by individual masks. Radiation approaches were conventionally fractionated definitive- (n13, median dose 66 Gy), adjuvant- (n31, median dose 60 Gy) or neoadjuvant radiotherapy (n22, median dose 40 Gy; additional postoperative dose escalation to median 68 Gy in five of these patients). Median radio-oncological treatment time was 39 days (range, 3–72 days).
Classification and statistics
Local treatment response was evaluated six to eight weeks after treatment by clinical examination and in recent years with ultrasonography and/or computed tomography (CT) or magnetic resonance imaging (MRI). The treatment response was classified as complete remission (CR, requiring no detectable disease), partial remission (PR, tumor mass reduction of at least 50%), no response (NR, less than 50% tumor mass reduction) or as progressive disease (PD). Toxicity was assessed using the Acute Radiation Morbidity Scoring Criteria and the Late Radiation Morbidity Scoring Scheme from the Radiation Therapy Oncology Group (RTOG/EORTC 2004). Statistical analyses were carried out with SPSS statistical package (SPSS Inc., Chicago, IL, USA) using log-rank test, χ2-test (Pearson, Fishers Exact Test), Kaplan-Meier's estimation and Cox-regression analyses (p-in < 0.05, p-out > 0.1, stepwise backwards). Significance was defined as p-value 0.05. All time estimates began with initial diagnosis.
Results
Survival
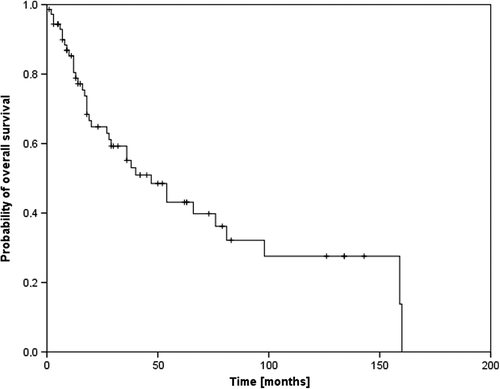
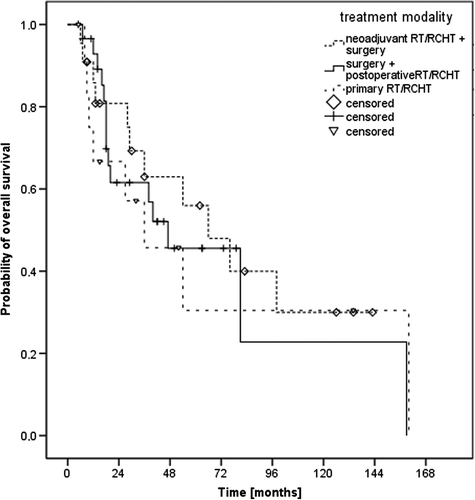
The median (mean) follow-up time was 29 (41) months with a range from 5 to 160 months. The median overall survival (OS) time was 54 months and the three- and five-year OS rates 59% and 46%, respectively (). In the subgroup of patients with T1-2 N0 disease, the median OS was 81 months and the five-year OS rate 52%. The impact of treatment regime (neoadjuvant vs. postoperative vs. definitive radio-oncological treatment) on OS is seen in , the corresponding median OS times were 66 months, 47 months and 36 months, respectively (p0.86). Assured information on the death of a patient was available in 29 cases. Causes of death were tumor progression in six patients, medical conditions in five patients, a secondary carcinoma in one patient and undocumented reasons in 17 patients.
Treatment response and local control
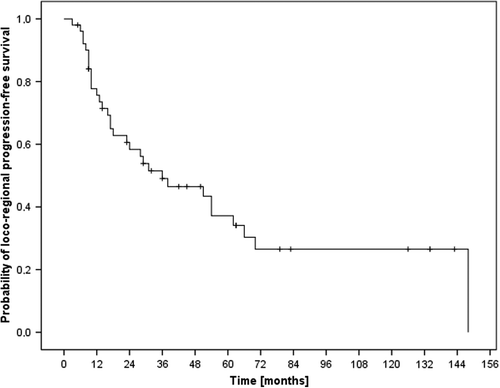
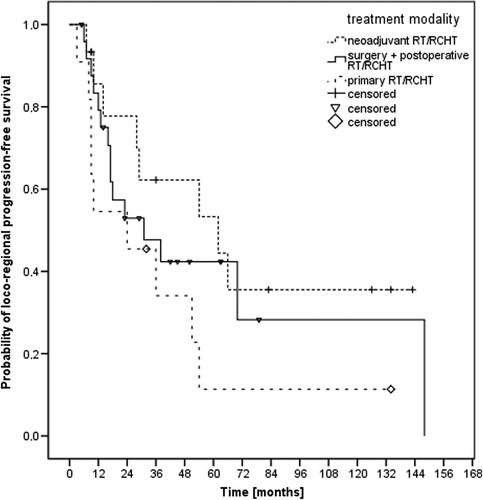
Forty-two patients (64%) achieved complete remission whereas 14 patients (21%) showed partial remission, three patients (4%) showed no change and two patients (3%) had progressive disease after treatment. No information on treatment response was available in five patients. The results of the different treatment modalities were found in . The median loco-regional progression-free survival (LRPFS) time was 36 months and the one- and two-year LRPFS rates 76% and 58%, respectively (). The impact of treatment regime (neoadjuvant vs. postoperative vs. definitive radio-oncological treatment) on LRPFS is seen in , the corresponding median LRPFS times were 62 months, 31 months and 24 months, respectively (p0.224).
Table II. Outcome according to treatment.
Prognostic factors
Upon univariate analysis (see ), significantly improved survival was found in patients without chronic alcohol (p0.005) or tobacco (p0.014) abuse in their history and with female gender (p0.025). In the subgroup of T1-2 N0 patients, univariate analysis revealed male gender as negative prognostic factor (p0.019) and showed borderline significance for smoking (p0.061). The results of the multivariate Cox-regression analysis were summarized in . In χ2-test, local recurrence showed a trend towards dependence on t-stage (Pearson, p0.082). Furthermore, neoadjuvant radiotherapy had a trend to be more commonly used in advanced T-stages (Pearson, p0.082), while surgery (Pearson, p0.167) and radiochemotherapy (Pearson, p0.21) were evenly distributed between T-stages. Fisher's exact test did not show significant influence of surgery (p0.293) or neoadjuvant radiotherapy (p0.534) on OS.
Table III. Univariate analysis.
Table IV. Multivariate analysis.
Toxicity
Acute low-grade skin and mucosal reactions were documented in all patients. Otherwise, acute toxicity grade III or IV was seen in 52% of patients. These acute high-grade toxicities were mainly mucositis and erythemas, partially requiring feeding tubes or treatment interruptions. None of the locally controlled patients reported grade IV or higher late toxicity.
Discussion
This retrospective analysis reports on the treatment results of 66 patients treated with concomitant platinum-based radiochemotherapy or immunotherapy and surgery (including tumor resection, neck dissection or suprahyoidal dissection) between 1980 and 2006 for squamous cell carcinoma of the mobile tongue or tongue margins (SCCT). The standard treatment is primary surgery with stage dependent postoperative radio(chemo)therapy, however for organ preservation preoperative radio(chemo)therapy is used as an individual approach. This analysis evaluates the treatment outcome at our institution as well as patterns of failure. Since different treatment regimes were used in this patient collective, the result comparison is limited. Still, the results might help finding ways to improve prognosis, morbidity and mortality in patients with SCCT.
Recently, Shim et al. reported on 86 patients treated with curative surgery with and without radiotherapy for oral tongue squamous cell carcinoma (T1-2 N0-1) [Citation15]. The achieved five-year OS was 80.8%. In a comparable cohort (T1-2 N0) Al-Rajhi et al. reported on a five-year OS of 71% [Citation16]. In the analysis by El-Husseiny et al. on squamous cell carcinoma of the oral tongue (T1-4 N0-3) the reported five-year OS was 65% [Citation17]. Rusthoven et al. described a five-year OS of 60.9% in patients treated for T1-2 N0 oral tongue squamous cell carcinoma [Citation18]. In the report by Rusthoven et al. on 50 patients treated for oral tongue cancer, the two-year OS was 65% [Citation19]. These superior results in comparison to ours might be explained by the high counts of T2- and T3- as well as N2-stages in our cohort, while the subgroup of T1-2 N0 disease was small and thereby limited statistical power. Regional control could decrease from 85.4% in node negative patients to 45.2% in node positive patients as recently reported by Goldstein et al. on 273 patients with squamous cell carcinoma of the oral tongue [Citation20]. Furthermore, they found nodal disease to be the only significant multivariate predictor for regional recurrence. The five- and 10-year relapse-free survival in the cohort of El-Husseiny et al. was comparable to results published by Lepeyre et al. on combined percutaneous radiotherapy and brachytherapy in T1-4 N squamous cell carcinoma of the oral cavity [Citation13]. Since new radiation techniques as intensity modulated radiotherapy are replacing older techniques, the risk of poor target delineation increases [Citation21,Citation22], while on the other hand, toxicity might be reduced. Recently, Damast and colleagues reported on four patients with marginal recurrences after selective targeting IMRT reminding readers to careful and accurate delineation of target volumes [Citation23].
Regarding possibly prognostic factors, Shim et al. reported higher tumor grade and invasion depth / 0.5 cm to be associated with shorter five-year disease-free and OS in multivariate analysis. T-stage was neither prognostic in uni- nor in multivariate analysis [Citation15]. El- Husseiny et al. published invaded resection margins and smoking as factors for shorter relapse-free and OS in multivariate analysis, while chewing tobacco influenced the relapse-free survival only [Citation17]. In the cohort published by Rusthoven et al. T2 disease and younger age were associated with an increased hazard for death in multivariate analysis, but there was no difference between oral tongue and other oral cavity subsites in this multivariate analysis [Citation18]. In contrast, our results did not show worse results in case of higher tumor grading and multivariate analysis did not show T-stage to significantly influence OS. Furthermore, the data from Princess Margaret Hospital revealed only pathologic N classification to be an independent predictor for OS as well as disease free survival [Citation20].
Conclusion
No statistically significant differences between treatment modalities were identified, but one should keep in mind that organ preservation plays a major role for the quality of patients’ life. Furthermore, none of the locally controlled patients reported grade IV or higher late toxicity. Still, tumor recurrence is common, especially in advanced tumor stage. Interdisciplinary concepts, further increasing the chance of tumor control are warranted.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Howell RE, Wright BA, Dewar R. Trends in the incidence of oral cancer in Nova Scotia from 1983 to 1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95:205–12.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225–49.
- Rosenquist K. Risk factors in oral and oropharyngeal squamous cell carcinoma: A population-based case-control study in southern Sweden. Swed Dent J Suppl 2005:1–66.
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 2000;92:709–20.
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: A systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467–75.
- Barry CP, Katre C, Papa E, Brown JS, Shaw RJ, Bekiroglu F, et al. De-escalation of surgery for early oral cancer – is it oncologically safe?Br J Oral Maxillofac Surg Epub 2012 Mar 21.
- Petera J, Matula P, Paluska P, Sirak I, Macingova Z, Kasaova L, et al. High dose rate versus low dose rate brachytherapy in the treatment of tongue carcinoma – a radiobiological study. Neoplasma 2009;56:163–8.
- Kobayashi Y, Karasawa K, Komiya Y, Hanyu N, Okamoto M, Chang T-C, et al. Therapeutic results for 100 patients with cancer of the mobile tongue treated with low dose rate interstitial irradiation. Anticancer Res 2007;27:1689–92.
- Yuen AP-W, Ho CM, Chow TL, Tang LC, Cheung WY, Ng RW-M, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck 2009;31:765–72.
- Lim YC, Lee JS, Koo BS, Kim S-H, Kim Y-H, Choi EC. Treatment of contralateral N0 neck in early squamous cell carcinoma of the oral tongue: Elective neck dissection versus observation. Laryngoscope 2006;116:461–5.
- D’Cruz AK, Siddachari RC, Walvekar RR, Pantvaidya GH, Chaukar DA, Deshpande MS, et al. Elective neck dissection for the management of the N0 neck in early cancer of the oral tongue: Need for a randomized controlled trial. Head Neck 2009;31:618–24.
- Kimura Y, Fuwa N, Kamata M, Matsumoto A, Nakamura PK, Nakamura T, et al. Stage I and II mobile tongue carcinomas treated by external radiation and gold seed implantation. Acta Oncol 2003;42:763–70.
- Lapeyre M, Bollet MA, Racadot S, Geoffrois L, Kaminsky M-C, Hoffstetter S, et al. Postoperative brachytherapy alone and combined postoperative radiotherapy and brachytherapy boost for squamous cell carcinoma of the oral cavity, with positive or close margins. Head Neck 2004;26: 216–23.
- Sturgis EM, Moore BA, Glisson BS, Kies MS, Shin DM, Byers RM. Neoadjuvant chemotherapy for squamous cell carcinoma of the oral tongue in young adults: A case series. Head Neck 2005;27:748–56.
- Shim SJ, Cha J, Koom WS, Kim GE, Lee CG, Choi EC, et al. Clinical outcomes for T1-2N0-1 oral tongue cancer patients underwent surgery with and without postoperative radiotherapy. Radiat Oncol 2010;5:43.
- Al-Rajhi N, Khafaga Y, El-Husseiny J, Saleem M, Mourad W, Al-Otieschan A, et al. Early stage carcinoma of oral tongue: Prognostic factors for local control and survival. Oral Oncol 2000;36:508–14.
- El-Husseiny G, Kandil A, Jamshed A, Khafaga Y, Saleem M, Allam A, et al. Squamous cell carcinoma of the oral tongue: An analysis of prognostic factors. Br J Oral Maxillofac Surg 2000;38:193–9.
- Rusthoven K, Ballonoff A, Raben D, Chen C. Poor prognosis in patients with stage I and II oral tongue squamous cell carcinoma. Cancer 2008;112:345–51.
- Rusthoven KE, Raben D, Song JI, Kane M, Altoos TA, Chen C. Survival and patterns of relapse in patients with oral tongue cancer. J Oral Maxillofac Surg 2010;68:584–9.
- Goldstein DP, Bachar GY, Lea J, Shrime MG, Patel RS, Gullane PJ, et al. Outcomes of squamous cell cancer of the oral tongue managed at the Princess Margaret Hospital. Head Neck 2012;5:797–803.
- Daly ME, Le Q-T, Kozak MM, Maxim PG, Murphy JD, Hsu A, et al. Intensity-modulated radiotherapy for oral cavity squamous cell carcinoma: Patterns of failure and predictors of local control. Int J Radiat Oncol Biol Phys 2011;80:1412–22.
- Jeanneret-Sozzi W, Moeckli R, Valley J-F, Zouhair A, Ozsahin EM, Mirimanoff R-O. The reasons for discrepancies in target volume delineation: A SASRO study on head-and-neck and prostate cancers. Strahlenther Onkol 2006;182:450–7.
- Damast S, Wolden S, Lee N. Marginal recurrences after selective targeting with intensity-modulated radiotherapy for oral tongue cancer. Head Neck 2012;34:900–6.