To the Editor,
10–30% of patients diagnosed with medulloblastoma develop extraneural metastases (ENM), of which bone metastases are the most common [Citation1]. Median survival is approximately eight months after diagnosis of ENM, [Citation2], and efficient treatment strategies are urgently needed.
The hedgehog (Hh) signaling pathway is important in the regulation of cerebellar maturation and development, and constitutive activation of this pathway occurs in about 30% of human medulloblastomas. It is significant that Hh pathway activation occurs in over 50% of adult medulloblastomas [Citation3,Citation4]. Vismodegib (GDC-0449) is a Hh signaling inhibitor that has shown promising preclinical efficacy in medulloblastoma [Citation5]. Only one case in which medulloblastoma was treated with vismodegib has been published [Citation6].
3′-Deoxy-3′-[18F]-fluorothymidine positron emission tomography (FLT PET) correlates well with proliferative activity in many cancers, including brain tumors [Citation7–9]. It is also possible to use FLT PET to quantify bone marrow response during radiotherapy [Citation10] and chemotherapy [Citation11], while sodium [18F] fluoride PET (NAF PET) is superior to conventional technetium bone scintigraphy for the detection and evaluation of skeletal metastases [Citation12–14].
The case
A male patient was 38 years of age in 2009 when diagnosed with a nodular desmoplastic medulloblastoma, with bilateral manifestation in the cerebellum. Surgery was almost radical, with a residual tumor of approximate size 1 cm in the transverse sinus. The patient received adjuvant craniospinal radiotherapy followed by eight courses of adjuvant temozolomide therapy.
Disseminated ENM was diagnosed in November 2010 during investigation of skeletal pain, and was confirmed by bone marrow biopsy. The patient received second-line therapy, according to a dose-reduced version of the HIT 2000 protocol, which included vincristine, cyclophosphamide, methotrexate, carboplatin and etoposide. The side effects of the treatment were unacceptable, and treatment was switched to metronomic antiangiogenic chemotherapy, as described by Kieran et al. [Citation15] and Buchroitner et al. [Citation16]. Autologous stem-cell transplantation was discussed but considered of no benefit, due to the patient's insufficient treatment response to chemotherapy.
The disease recurred in December 2011 in the thoracic and lumbar spine causing severe radiating pain, 10 out of 10 on the visual analogue scale (VAS). This initially required sedation and treatment with intravenous infusions of ketamine and morphine hydrochloride at an intensive care unit. Subsequently, very high doses of oral analgesics, with daily doses of 150 mg ketamine, 90 mg methadone, 3600 mg gabapentin and 60 mg morphine hydrochloride were given, together with 9 mg of betamethasone and benzodiazepines. NAF PET/computed tomography (CT) showed generalized uptake in the bone (). The patient had previously received radiation treatment to the entire spine, as described above, but FLT PET/CT showed that activity in the bone marrow was still high and heterogeneous, even in the vertebral column (). The spleen was enlarged and displayed intense proliferative activity. This was consistent with extramedullary hematopoiesis in the spleen caused by severe bone marrow suppression [Citation17], mainly as a result of tumor infiltration into the bone marrow compartment. A secondary cause was the craniospinal irradiation described earlier. Retreatment with palliative radiotherapy to the spine was given to alleviate the pain with a short-course fractionation using a single posterior field.
Figure 1. Sodium [18F] fluoride PET (NAF PET) response. Four repeated NAF PET/CT scans in coronal view before (Figure 1A), and 2 weeks (Figure 1B), 2 months (Figure 1C) and 5 months (Figure 1D) after the start of vismodegib treatment. A generalized uptake of NAF is seen in Figure 1A. The intensity of uptake in the pelvic and hip bone has decreased in Figure 1B, and remains unchanged in Figure 1C. The uptake in the skeleton is still heterogeneous in Figure 1D, and has increased slightly.
![Figure 1. Sodium [18F] fluoride PET (NAF PET) response. Four repeated NAF PET/CT scans in coronal view before (Figure 1A), and 2 weeks (Figure 1B), 2 months (Figure 1C) and 5 months (Figure 1D) after the start of vismodegib treatment. A generalized uptake of NAF is seen in Figure 1A. The intensity of uptake in the pelvic and hip bone has decreased in Figure 1B, and remains unchanged in Figure 1C. The uptake in the skeleton is still heterogeneous in Figure 1D, and has increased slightly.](/cms/asset/d04cd912-b401-463a-a15e-12263795e39e/ionc_a_724537_f0001_b.jpg)
Figure 2. [18F]-fluorothymidine positron emission tomography (FLT PET) response. Four repeated FLT PET/CT scans in coronal view before (Figure 2A), and 2 weeks (Figure 2B), 2 months (Figure 2C) and 5 months (Figure 2D) after the start of vismodegib treatment. The uptake of FLT in the bone marrow is heterogeneous in Figure 2A. The spleen is enlarged and FLT uptake is intense. Figure 2B and C show a considerable reduction in FLT activity in bone marrow. The spleen is still enlarged with a high FLT uptake. A slight increase in the uptake can be seen in Figure 2D, heterogeneously distributed in the ribs, proximal humeri and femora. Uptake remains low in the spine. The sizes of the liver and spleen have decreased.
![Figure 2. [18F]-fluorothymidine positron emission tomography (FLT PET) response. Four repeated FLT PET/CT scans in coronal view before (Figure 2A), and 2 weeks (Figure 2B), 2 months (Figure 2C) and 5 months (Figure 2D) after the start of vismodegib treatment. The uptake of FLT in the bone marrow is heterogeneous in Figure 2A. The spleen is enlarged and FLT uptake is intense. Figure 2B and C show a considerable reduction in FLT activity in bone marrow. The spleen is still enlarged with a high FLT uptake. A slight increase in the uptake can be seen in Figure 2D, heterogeneously distributed in the ribs, proximal humeri and femora. Uptake remains low in the spine. The sizes of the liver and spleen have decreased.](/cms/asset/62543678-9e7d-48cd-9720-a0059d437655/ionc_a_724537_f0002_b.jpg)
No documented treatment option remained open, but we were aware of a case report that described tumor regression induced by a Hh pathway inhibitor in a patient with metastasized medulloblastoma [Citation6]. We decided to analyze the patient's tumor for Hh pathway activity (as described in Supplementary “Materials and methods”, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.724537) before considering treatment with vismodegib. This drug is an inhibitor of Smoothened (SMO), a G-protein-coupled receptor (GPCR)-like molecule that plays a key role in Hh signaling.
Real-time-PCR analysis of two separate parts of the patient's primary tumor taken before treatment revealed strongly upregulated expression of Hh pathway target genes. The two direct Hh target genes, GLI1 and patched homologue 2 (PTCH2), were expressed at levels 70–140 times higher in the medulloblastoma tumor cells than in Daoy cells ( and B). Daoy is a well-characterized human medulloblastoma cell line with active Hh signaling [Citation18], and the mRNA levels of GLI1 are comparable to the levels in primary medulloblastomas classified as belonging to the Hh pathway active subgroup [Citation19].
Figure 3. Hedgehog signaling in the tumor compared to Daoy cells. Hedgehog (Hh) pathway activation in medulloblastoma tumor cells compared to activation in the Daoy cell line. The mRNA levels (assayed by real-time RT-PCR) of GLI1 and PTCH2 are strongly upregulated and the GLI2 mRNA level moderately upregulated, relative to the levels in the Daoy cell line. Shown are two biological replicates (Figure 3A and B) measured in technical triplicates, which were analyzed using the 2–ΔΔCT method using the housekeeping genes RPLP0 and 18S as references. Stdev (ct) ≤ 0.64.
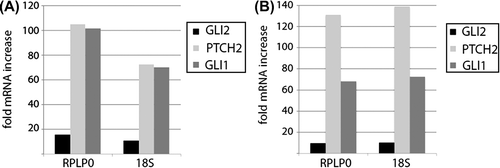
The expression level of the Hh pathway member GLI2, which is not a direct transcriptional target of the pathway, was also increased but to a much lesser extent ( and B). The highly increased Hh target gene expression suggests that tumor cell growth is driven by dysregulated Hh signaling.
Treatment with vismodegib was started in January 2012 with a continuous dose of 150 mg daily [Citation20,Citation21]. After two weeks, there was a clear and general reduction in proliferative activity as assessed by FLT PET/CT (), indicating that the effect was general and not restricted to the treatment field of the radiotherapy. After eight weeks (), the intensity and distribution of FLT were the same as they had been in the FLT PET/CT examination performed after two weeks of treatment. The NAF PET/CT activity in the pelvic bone (–C) fell slightly during this period.
Pancytopenia also improved during treatment with vismodegib. The need for erythrocyte transfusions decreased from one to two times weekly, with a baseline hemoglobin (Hb) of 64 g/L, to once every second month. In June 2012 the Hb level was above 100 g/l. Platelets were transfused every second week before treatment with vismodegib, while the last transfusion was given at the beginning of February. This was accompanied by a small increase of leucotyes from slightly below 3×109/L to slightly above that level. The improved status and well-being of the patient during the same time period can be seen in the improvement in albumin, which increased from 26–32 g/L to 40–44 g/L (reference range, 36–45 g/L).
The only side effect of vismodegib so far has been slight myalgia in the lower legs. The pain, including myalgia, has decreased to VAS 1, allowing a considerable reduction in the doses of analgesics. Five months after the start of treatment with vismodegib, in June 2012, the daily doses of methadone and gabapentin had been reduced to 30 mg and 900 mg, respectively, while ketamine and betamethasone had been completely tapered off. NAF PET/CT () in June 2012, after five months of treatment with vismodegib, showed a slight increase in activity from that of the previous scan (), as was also shown by FLT PET/CT (). There was a slight increase in activity bilaterally in the proximal femora and humeri and also in the ribs from the activity recorded in the previous examination (), while the activity in the previously irradiated vertebral column was still low (). The spleen had been reduced in size considerably, and the liver to a lesser extent.
Early disease progression based on the most recent PET/CT examinations (Figures 1D and 2D) cannot be ruled out by these observations. However, the increase in activity did not include the previously irradiated vertebral column, from which the patient's initial severe neurological symptoms originated. In addition, the reduction in the size of the spleen suggests that the extramedullary hematopoietic activity had fallen [Citation17], which is consistent with an improved bone marrow function. Finally, and most importantly, a substantial clinical and hematological improvement was evident, confirming that a partial restoration of bone marrow has occurred. As treatment with vismodegib was started the platelet level was 14×109/L. After two months of treatment it was 19×109/L (corresponding to PET/CT examinations represented by Figures 1C and 2C), while the platelet level after five months of treatment was 85×109/L (corresponding to PET/CT examinations represented by Figures 1D and 2D) and 102×109/L the same month, but two weeks after the examinations. The slight increase in isotope activity was therefore interpreted to indicate vitalized bone marrow after a long period of bone marrow suppression secondary to infiltrating tumor cells.
Compared to a previous case report of metastasized medulloblastoma treated with vismodegib [Citation6], the patient described here experienced a substantial improvement of pancytopenia. The previously reported patient succumbed to the disease approximately five months after the start of treatment with vismodegib [Citation6], while the patient described here has performance status grade 1 (according to the WHO- ECOG criteria) after five months of treatment (June 2012). A pathway that may explain differences in outcome among patients treated with inhibitors of Hh signaling requires a mutation of SMO-WT to SMO-D473H [Citation22], existing either already before treatment start or as a feature that arose during the course of treatment.
In conclusion, we present a case of a patient with metastasized medulloblastoma, refractory to treatment with conventional cytostatic drugs, but showing a remarkable clinical and hematological response to vismodegib. The side effects are mild, and the patient is still in a good condition 19 months after the diagnosis of ENM medulloblastoma and five months after introducing vismodegib.
Supplementary “Materials and methods”
Download PDF (21.2 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Spencer CD, Weiss RB, Van Eys J, Cohen P, Edwards B. Medulloblastoma metastatic to the marrow. Report of four cases and review of the literature. J Neurooncol 1984;2: 223–35.
- Mazloom A, Zangeneh AH, Paulino AC. Prognostic factors after extraneural metastasis of medulloblastoma. Int J Radiat Oncol Biol Phys 2010;78:72–8.
- Al-Halabi H, Nantel A, Klekner A, Guiot MC, Albrecht S, Hauser P, et al. Preponderance of sonic hedgehog pathway activation characterizes adult medulloblastoma. Acta Neuropathol 2011;121:229–39.
- Remke M, Hielscher T, Northcott PA, Witt H, Ryzhova M, Wittmann A, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol 2011;29:2717–23.
- Dlugosz AA, Talpaz M. Following the hedgehog to new cancer therapies. N Engl J Med 2009;361:1202–5.
- Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med 2009;361: 1173–8.
- Chalkidou A, Landau DB, Odell EW, Cornelius VR, O’Doherty MJ, Marsden PK. Correlation between Ki-67 immunohistochemistry and 18F-Fluorothymidine uptake in patients with cancer: A systematic review and meta-analysis. Eur J Cancer Epub2012 May 31.
- Price SJ, Fryer TD, Cleij MC, Dean AF, Joseph J, Salvador R, et al. Imaging regional variation of cellular proliferation in gliomas using 3’-deoxy-3’-[18F]fluorothymidine positron-emission tomography: An image-guided biopsy study. Clin Radiol 2009;64:52–63.
- Backes H, Ullrich R, Neumaier B, Kracht L, Wienhard K, Jacobs AH. Noninvasive quantification of 18F-FLT human brain PET for the assessment of tumour proliferation in patients with high-grade glioma. Eur J Nucl Med Mol Imaging 2009;36:1960–7.
- Agool A, Slart RH, Thorp KK, Glaudemans AW, Cobben DC, Been LB, et al. Effect of radiotherapy and chemotherapy on bone marrow activity: A 18F-FLT-PET study. Nucl Med Commun 2011;32:17–22.
- Vanderhoek M, Juckett MB, Perlman SB, Nickles RJ, Jeraj R. Early assessment of treatment response in patients with AML using [(18)F]FLT PET imaging. Leuk Res 2011;35:310–6.
- Cook GJ. PET and PET/CT imaging of skeletal metastases. Cancer Imaging 2010;10:1–8.
- Withofs N, Grayet B, Tancredi T, Rorive A, Mella C, Giacomelli F, et al. (1)(8)F-fluoride PET/CT for assessing bone involvement in prostate and breast cancers. Nucl Med Commun 2011;32:168–76.
- Iagaru A, Mittra E, Dick DW, Gambhir SS. Prospective evaluation of (99m)Tc MDP scintigraphy, (18)F NaF PET/CT, and (18)F FDG PET/CT for detection of skeletal metastases. Mol Imaging Biol 2012;14:252–9.
- Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol 2005;27: 573–81.
- Buchroithner J, Nußbaumer K, Pichler S, Weis S. Anti- angiogenetic metronomic chemotherapy in an adult with recurrent medulloblastoma with spinal metastases: A case report. Mag Eur Med Oncol 2010;3(Suppl 3):25–7.
- Kim CH. Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med 2010;1:13–9.
- Di Marcotullio L, Ferretti E, De Smaele E, Argenti B, Mincione C, Zazzeroni F, et al. REN(KCTD11) is a suppressor of hedgehog signaling and is deleted in human medulloblastoma. Proc Natl Acad Sci U S A 2004;101:10833–8.
- Bar EE, Chaudhry A, Farah MH, Eberhart CG. Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol 2007;170:347–55.
- Lorusso PM, Jimeno A, Dy G, Adjei A, Berlin J, Leichman L, et al. Pharmacokinetic dose-scheduling study of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors. Clin Cancer Res 2011;17:5774–82.
- LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res 2011; 17:2502–11.
- Metcalfe C, de Sauvage FJ. Hedgehog fights back: Mechanisms of acquired resistance against smoothened antagonists. Cancer Res 2011;71:5057–61.