Palliative treatments are often given to patients with a reduced performance status, limited life expectancy, and pre-existing symptom burden. Therefore, they should ideally relieve symptoms quickly, effectively and durably, while also causing minimum toxicity [Citation1]. Toxicity is largely due to the irradiation of normal organs, and in some cases may be enhanced by systemic therapy [Citation2]. The widespread availability of advanced radiotherapy technologies, including intensity-modulated radiotherapy (IMRT), provides greater opportunities for sparing organs-at-risk (OAR) and increasing the therapeutic ratio of our treatments [Citation3]. However, the use of IMRT for palliation faces significant challenges, including perceptions that it is inevitably time and labor intensive and that it may be an inefficient or inappropriate use of resources, a current lack of high-level supportive clinical evidence, and weaknesses in the implementation of new technologies and treatment techniques [Citation4]. Serious efforts are required to overcome these barriers and make available the potential advantages of widely available technologies to all patients who may benefit.
In this report, we describe the introduction of volumetric IMRT (RapidArc, Varian Medical Systems, Palo Alto, CA, USA) for palliating large- volume pelvic bone metastases, which we defined as those including the hemi- or full pelvis, either with or without inclusion of one or both hips and/or lower lumbar spine. The rationale for considering this an appropriate site for organ-sparing radiotherapy included: 1) standard conventional antero-posterior/postero-anterior (APPA) or box techniques often result in large volumes of bowel receiving the prescription dose, and the concave pelvic target volume encircling normal organs (bowel, bladder and genital region) lends itself to IMRT and the use of standard solutions to optimize planning target volume (PTV) coverage and spare central OARs; 2) gastrointestinal toxicity occurs frequently after large-field pelvic palliative RT [Citation5,Citation6]; 3) indications for palliation of bone metastases often arise during ongoing systemic therapy; 4) the requirements for pelvic re-irradiation may increase as systemic therapies improve; and 5) volumetric modulated arc delivery has been shown to be time-efficient and is well-suited to the fast irradiation of large volumes [Citation7]. In order to make this a practical solution for selected patients in a resource constrained environment, a standardized workflow addressing all steps of the IMRT process from delineation to quality assurance (QA) and treatment delivery was developed. We describe the technical details of this approach, and the initial clinical experience.
Materials and methods
A multi-disciplinary team consisting of a physician, physicist and technologist designed the following workflow and technique for implementation in an academic medical center with six linear accelerators treating approximately 2750 patients/year.
Workflow: Treatment planning and quality assurance
A standard planning computed tomography (CT) scan (no contrast, slice thickness 2.5 mm) is performed for treatment planning with the patient supine, lying on a mattress. Arms are placed either above the head or crossed on the upper body, away from the pelvis. If the former is not comfortable for patients, arms are placed alongside the body. After importing the CT into the treatment planning system (Eclipse v8.9 and subsequently v10.0, Varian Medical Systems Inc., Palo Alto, USA), a standard structure set is attached to it. The relevant part of the pelvic bone [in this instance clinical target volume (CTV) = gross tumor volume (GTV)] is delineated by a technologist using a semi-automated CT thresholding tool, and post-processing functions available in the planning system. The contours are checked by a physician and manually edited if necessary (e.g. at sites of soft-tissue extension). As the treatment is delivered with online image-guidance, an isotropic margin of 5 mm is added to create the PTV. In cases where the hip/proximal femur is included in the CTV, a 10 mm PTV margin may be applied to these particular structures in order to allow for easier patient set-up without compromising bowel sparing. Where necessary the PTV was cropped to within 5 mm of the patient body contour.
OARs are defined as the abdominal contents, including the bowel and other central organs (e.g. bladder and genitalia), and these are rapidly delineated as a single structure using a very large contouring brush that automatically avoids the PTV and is automatically cropped to the body contour. For the purposes of more accurately reporting the dose to the abdominal cavity itself in the present report, this structure was retrospectively modified to extend to the anterior border of the abdominal cavity, inner bony contour of the pelvis (i.e. within the PTV), 5 mm above and below the PTV, and to exclude the external genital organs. A ring structure is created around the PTV to facilitate a fall off in dose outside the PTV. This was used in six of seven cases described in this report, and is now routine practice. Standard dose prescription is a single fraction of 8 Gy, although some variation is permitted, e.g. in patients undergoing re-irradiation.
The PTV, OAR and ring structure are used for dose optimization, which is performed with minimal interaction between the technologist and the planning system. The order of priority in the optimization (most important first) is as follows: 1) PTV (1–2 upper and 1 lower objective with priorities 110–140); 2) OAR (2–3 upper objectives, priorities 50–130); and 3) ring structure (4–7 upper objectives, priorities 70–100). In most cases, delivery is performed using two arcs, but it can be more depending, e.g. on the total number of monitor units. The treatment plan is reviewed by a physician, and if clinically acceptable checked by a second technologist, not involved in creating the plan, and a physicist. There is a final check by a technologist prior to exporting the plan to the treatment machine. Quality assurance is performed with the Matrixx ionization chamber array (IBA Dosimetry GmbH, Schwarzenbruck, Germany) using a gamma evaluation with dose and distance tolerances of 3% or 4% and 2 mm, respectively. In some cases plans may be measured after the treatment has been delivered. This has been permitted on the basis of extensive prior satisfactory experience with RapidArc QA (> 2000 measured plans), relatively modest prescription dose, delivery under monitored conditions (e.g. jaw and multi-leaf collimator positions) and a regular machine QA program. If available, time-efficient QA measurement methods (e.g. EPID dosimetry) may offer logistic advantages.
Workflow: Treatment delivery
All patient treatments follow standard procedures, including ensuring patient comfort and adequate analgesia. Initial patient positioning is performed using skin marks. Thereafter, online imaging and four-dimensional (4D) couch shift (translational corrections + couch rotation) is performed using orthogonal kilovolt (kV) images and matching on bony structures. In some cases, (e.g. where kV image match is sub-optimal or for verification of the position of both hips) a CBCT may be made to confirm patient position.
Results
summarizes the treatment plan characteristics and delivery details for the first seven patients. For patient 1, data for a conventional plan made with two opposing fields is included for illustrative purposes () and shows that the mean dose to the bowel was about twice that in the corresponding RapidArc plan. The set-up time for patient 6 was prolonged by the need to re-position in order to correct a pitch in the pelvis.
Table I. Treatment characteristics. Treatment plan and delivery characteristics for the first seven patients treated using volumetric modulated arc therapy. For illustrative purposes, data for a treatment plan made with an anterior-posterior/posterior-anterior (APPA) beam arrangement is shown for patient 1.
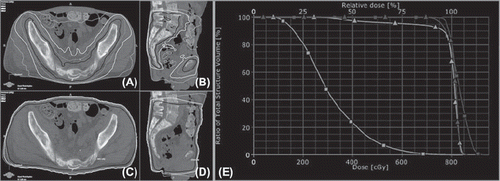
Figure 1. Conformal, intensity modulated RapidArc (RA) treatment plan (A + B), and conventional anterior-posterior/posterior-anterior (APPA) field arrangement (C + D), demonstrating the dose distribution and the bowel sparing that can be achieved with RA (moving from outside to inside: isodose lines shown for 2, 4, 6 and 7.6 Gy). On the right the dose volume histogram (E) illustrates that in both cases the PTV is covered with at least 7 Gy (RA = squares and APPA = triangles) but the RA plan delivers much less dose to the abdominal contents (light color line, squares) than an APPA field arrangement (light color line, triangles).
Quality assurance
For a dose difference of 3% with a distance to agreement (DTA) of 2 mm, we found a mean gamma of 0.47 with a gamma > 1 for on average 6.5% (range 0–24.8%) of the data points. For a dose difference of 4% and a DTA of 2 mm the mean gamma index was 0.38 and mean gamma > 1 was on average 2.3% (range 0–8.7%).
Discussion
The use of newer treatment techniques for organ sparing palliative radiotherapy has been impeded by the belief that they are time consuming, difficult or perhaps even unnecessary for patients with a poor prognosis. We have described a simple, technologist-led workflow using minimal additional personnel, to enable the routine irradiation of selected patients with large-volume pelvic bone metastases using bowel-sparing volumetric modulated arc therapy. The clinical treatment plans provided good target coverage and achieved considerable reduction in OAR dose. Although not necessary in these patients, it is possible to complete the entire process from CT acquisition to treatment in one day. The time required to import the planning CT, complete IMRT treatment planning and perform initial checking is approximately two hours, compared to about one hour or less for a conventional plan. We consider the average treatment time to be acceptable for this initial phase and based on prior experience with implementing new techniques expect this to fall. In selected patients larger PTV margins (e.g. a uniform 10 mm margin for those in pain) and optimal pain management may help to facilitate set-up and enhance efficiency. Furthermore, the use of high dose rate treatment beams is expected to result in shorter delivery times. The average QA results were acceptable but for individual cases we have seen some deviations that are larger than desirable. The reason for this is currently uncertain, however, at the moment we hypothesize that it might have been due to the large treatment volumes resulting in irradiation of the electronics of the QA device and the amount of modulation in some of the plans.
In conclusion, the implementation of a straightforward and efficient workflow has enabled us to make volumetric IMRT routinely available for large-field palliative pelvic bone treatments. The ability to achieve this in a busy clinical environment is a prerequisite for evaluating new radiotherapy techniques in the palliative setting and for facilitating prospective clinical trials designed to improve the therapeutic ratio of palliative radiotherapy.
Declaration of interest: The Department of Radiation Oncology, VU University Medical Center has research collaborations with Varian Medical Systems Inc., USA. MD has received travel support ± honoraria from Varian Medical Systems Inc., and Brainlab AG. SS has received travel support and honoraria from Varian Medical Systems Inc. and served as a consultant. BS has received travel support and honoraria from Varian Medical Systems Inc., and Brainlab AG and has served as a consultant. WV has received travel support and honoraria from Varian Medical Systems Inc.
References
- van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy – new approaches. Semin Oncol 2011; 38:443–9.
- Peters NA, Richel DJ, Verhoeff JJ, Stalpers LJ. Bowel perforation after radiotherapy in a patient receiving sorafenib. J Clin Oncol 2008;26:2405–6.
- McIntosh A, Dunlap N, Sheng K, Geezey C, Turner B, Blackhall L, et al.Helical tomotherapy-based STAT RT: Dosimetric evaluation for clinical implementation of a rapid radiation palliation program. Med Dosim 2010;35:280–6.
- Bak K, Dobrow MJ, Hodgson D, Whitton A. Factors affecting the implementation of complex and evolving technologies: Multiple case study of intensity-modulated radiation therapy (IMRT) in Ontario, Canada. BMC Health Serv Res 2011;11:178.
- Berg RS, Yilmaz MK, Høyer M, Keldsen N, Nielsen OS, Ewertz M. Half body irradiation of patients with multiple bone metastases: A phase II trial. Acta Oncol 2009;48:556–61.
- Dennis K, Nguyen J, Presutti R, Deangelis C, Tsao M, Danjoux C, et al.Prophylaxis of radiotherapy-induced nausea and vomiting in the palliative treatment of bone metastases. Support Care Cancer 2012;20:1673–8.
- Matuszak MM, Yan D, Grills I, Martinez A. Clinical applications of volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys 2010;77:608–16.