Abstract
Background. Forkhead Box Protein 3 (FOXP3) is a marker for immunosuppressive CD4+CD25+ regulatory T cells (Tregs). We investigated whether there were significant numbers of FOXP3-positive Tregs in triple-negative breast cancer (TNBC) using immunohistochemistry, and whether the presence of FOXP3-positive Tregs was associated with other prognostic factors, such as stage or histologic grade. We investigated the number of tumor-infiltrating FOXP3-positive Tregs in formalin-fixed TNBC specimens obtained from patients who received palliative treatment between 1999 and 2007. Material and methods. Immunohistochemistry was used to assess the number of CD4+, CD25+, and FOXP3+ Tregs in tumor tissue and normal breast tissue from 86 TNBC patients. Univariate and multivariate analyses evaluated outcomes according to the number of FOXP3-positive Tregs. Results. Of the 86 tumor specimens, 22 (25.6%) expressed more than 15 FOXP3-positive Tregs per 10 high power fields in the peritumoral area. On multivariate analysis, staining showing ≥ 15 FOXP3-positive Tregs was an independent prognostic factor for overall survival and progression free survival with hazard ratios of 2.4 (95% CI 1.0–5.6; p = 0.049) and 2.0 (95% CI 1.1–3.6; p = 0.032), respectively. In TNBC, FOXP3-positive Tregs had stronger prognostic significance than did FOXP3-negative Tregs. The finding of improved survival associated with highly infiltrating FOXP3-positive Tregs in TNBC contrasted with several other types of solid cancer. Conclusion. TNBC may be differently driven by FOXP3 via an immune mechanism. The inclusion of FOXP3+ Tregs may help to improve prognostication for TNBC.
Triple-negative breast cancer (TNBC) is now commonly used to define breast cancer subtypes that are estrogen receptor (ER) negative, progesterone receptor (PR) negative, and HER2 negative according to the clinically available immunohistochemical (IHC) staining methods for these biomarkers. TNBCs comprise 10–15% of all breast cancers [Citation1] and are of pivotal clinical importance given the lack of effective therapeutic options, despite the worse outcomes following conventional treatment. The aggressive clinical course, poor prognosis, and lack of therapeutic options for this type of tumor have intensified current interest in this patient group [Citation2].
Regulatory T cells (Tregs) are a specialized subpopulation of T cells that suppress the activation of other immune cells, thereby maintaining systemic immune homeostasis. The precise mechanisms of the immune suppression remain unclear. Although T cells present the most important immunological response in tumor growth in the early stages of cancer, they become Tregs after chronic stimulation and interactions with tumor cells, promoting rather than inhibiting cancer development and progression.
Tregs may play an important role in suppressing this tumor-associated antigen-specific immunity. Higher numbers of Tregs have been found in the peripheral blood and tumor tissues of patients with a variety of tumors, including breast cancer [Citation3–5], ovarian cancer [Citation6], gastric cancer [Citation7,Citation8], esophageal cancer [Citation9], colorectal cancer [Citation10], melanoma [Citation11], hepatocellular carcinoma [Citation12,Citation13], and others [Citation14]. Some studies have found an association between high numbers of Tregs and poor clinical outcomes [Citation3,Citation4,Citation13,Citation15], but other studies have not found any relationship between Tregs and prognosis [Citation5,Citation9,Citation10].
Forkhead Box Protein 3 (FOXP3) represents the most specific marker for Tregs. Roncador et al. [Citation16] demonstrated that only approximately half of the CD4+CD25+ population expresses FOXP3. Thus, FOXP3-positive Tregs represent a more distinct T cell population than those described in previously published studies that relied on CD4+CD25+ profile alone. This implies that FOXP3 level, an indicator of Treg activity, might also be an indicator of breast tumorigenesis. Since invasion, size, and vascularity are prognostic parameters in breast cancer, the finding of an association between FOXP3 expression and these parameters suggests a role of FOXP3 as a marker of progression of breast carcinoma to an aggressive tumor phenotype.
Our prespecified hypothesis was that the numbers of FOXP3-positive Tregs would be clinically relevant in TNBC patients, with a high number correlating with a better or worse outcome. Given the proposed immune modulating effects of Tregs in patient tumor response, we attempted to correlate the number of Tregs within breast carcinoma tissue sections with other defined prognostic indicators of clinical outcome.
Material and methods
Patients
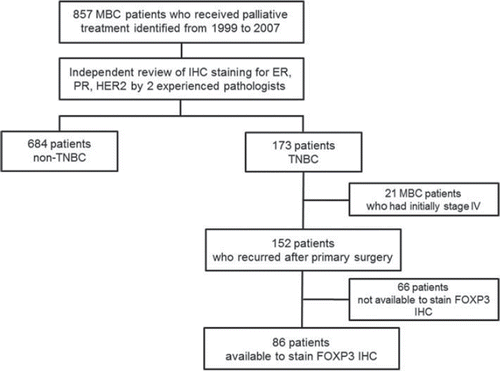
Between 1999 and 2007, 857 patients with metastatic breast cancer (MBC) received palliative chemotherapy, hormonal therapy, and/or targeted therapy at Samsung Medical Center. An experienced pathologist, who determined the expressions of CD4, CD25, and FOXP3 using IHC staining, reviewed all the pathologic specimens. Of these, 173 patients were identified as having ER/PR- negative and HER2-negative TNBC. Eighty-six patients had archival breast tumor samples available for IHC studies for CD4+, CD25+, and FOXP3. The present study was performed with same cohort as used by Cho et al. [Citation17]. We retrospectively obtained patients’ medical records, including demographic characteristics and clinical follow-up data. The patients were followed for a median of 73.5 months (range 24.2–120.0 months). This study was approved by the Samsung Medical Center Institutional Review Board.
CD4+, CD25+, and FOXP3 IHC and measurement
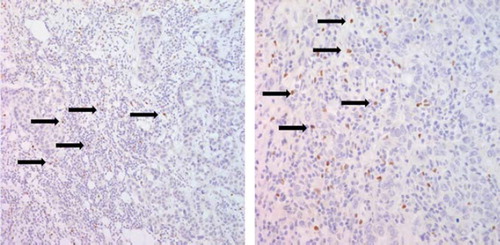
In this study, FOXP3, CD4, CD25 expression was evaluated using formalin-fixed, paraffin-embedded tumor sections. Tissue sections on glass slides were deparaffinized with xylene, hydrated in serially diluted alcohol, and then immersed in 3% hydrogen peroxide (H2O2) to quench endogenous peroxidase activity. Sections were subsequently processed in pH 9 Tris-EDTA (TE) buffer for 15 minutes in the microwave for antigen retrieval. Avidin and biotin were then applied consecutively to slides to eliminate endogenous biotin-related background staining, and the slides were incubated with primary antibodies for 60 minutes, rinsed with washing buffer three times, and incubated with biotinylated antibodies for FOXP3 (clone ab4728, 1:100 dilution; Abcam, Cambridge, UK), CD4 (clone 4B12, 1:100 dilution; DAKO, Glostrup, Denmark), and CD25 (clone NCL-CD25-305, 1:300 dilution; Novocastra, Newcastle, UK). After rinsing, the tissue sections were incubated with horseradish peroxidase- conjugated streptavidin for 20 minutes at room temperature. Slides were then washed, developed for 5 minutes with liquid 3,3’-diaminobenzidine, counter-stained with Meyer's hematoxylin, dehydrated, and mounted with Canada balsam for examination. Distilled water containing 0.1% Tween 20 was used as a rinsing solution. The presence or the absence of FOXP3 positive lymphocytes and quantification of FOXP3 positive Tregs was evaluated by an experienced pathologist who was blinded to the clinicopathologic data. At least 10 high power fields of peritumoral area were assessed. The presence of ≥ 15 FOXP3 positive Tregs, which was median value of the results, was considered as high FOXP3 expression [Citation3]. For the quantification of FOXP3, a status-blinded pathologist counted the absolute number of FOXP3-positive lymphocytes using an eyepiece graticule.
Statistical analyses
Recurrence-free survival (RFS) was measured from the day of curative surgery to the first day of documented recurrence. Progression-free survival (PFS) was measured from the first day of first-line palliative chemotherapy after recurrence to disease progression or death. Overall survival (OS) was measured from the day of first-line chemotherapy to death or the final follow-up day. In univariate analysis, independent sample t-tests and χ2-tests were used for continuous and categorical variables, respectively. The Kaplan-Meier product-limit method was used to estimate the RFS, PFS, and OS. The survival rates were compared using the log-rank test. Multivariate analysis of the independent prognostic factors for survival was performed using the backward stepwise (likelihood-ratio statistics based on the conditional parameter estimate) method of the Cox proportional hazard regression model with a 95% confidence interval (CI). We also incorporated the newly proposed Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK) criteria for tumor marker publications into this study [Citation18]. As recommended under the REMARK criteria, we included a diagram to describe the descriptive analysis of patient samples in this study.
Results
Patient characteristics and FOXP3 expression in breast cancer tumor cells
shows the patients’ cohort of the study. Of 857 patients with MBC who received palliative management at Samsung Medical Center between 1999 and 2007, 173 TNBCs were identified. Of the 173 patients, the number of relapsed patients after curative surgery was 152, and the remaining 21 patients had initial metastatic disease. Our final cohort included 86 patients who had available tissues to stain Tregs IHC. lists the frequency of prognostic clinicopathologic characteristics according to high or low expression of FOXP3 on immunostaining. FOXP3 staining was always localized in the nucleus of breast cancers (). Of the 86 tumor specimens, 22 patients (25.6%) were found to express FOXP3-positive Tregs (≥ 15) in the peritumoral area. Within the invasive tumor samples, there was a significant correlation between FOXP3-positive Tregs (≥ 15) and lymphovascular invasion (p = 0.032), adjuvant taxane use (p = 0.048), metastasis to local lymph nodes (p = 0.048), and metastasis to pleura (p = 0.008). Patients with tumors that showed staining of ≥ 15 FOXP3-positive Tregs had significantly longer OS (p = 0.001) and PFS (p = 0.036), but there was not a significant association with RFS (p = 0.495) ().
Table I. Correlation analyses between the number of FOXP3-positive Tregs and both clinicopathologic data for 86 TNBC patients.
CD4 and CD25 expression in TNBC
CD25 is not restricted to T cells and both Treg. In addition, activated effector T cell populations are CD25+ [Citation19], the CD4+CD25+ phenotype dose no solely represent the human Tregs. To exclude those CD4+ cell with intermediated to low levels of CD25 expression without immunoregulatory function [Citation20], the CD4+CD25highFOXP3+ phenotype was recommended for identifying the human Tregs. The CD4+ expression T cells were 31 of 94 patients and CD25high expression T cells were 57 of 94 patients. The CD4+CD25highFOXP3+ Tregs were 23 of 94, which showed CD4+ and CD25high expressions at the same time.
Prognostic significance of FOXP3 expression in TNBC
Univariate analysis of clinical and pathobiologic characteristics revealed that stage III tumors, adjuvant anthracycline use, and adjuvant taxane use were significantly associated with RFS (p = 0.007, p = 0.018, and p = 0.019, respectively; data not shown), whereas no prognostic variables were associated with OS except for being FOXP3- positive (). Analysis of OS and PFS according to the presence of FOXP3 using the log-rank test revealed a significantly better prognosis for patients with positive staining (p = 0.036 for PFS and p = 0.001 for OS; and B). The group with TNBC and high expression of FOXP3 had a significantly better prognosis than did the group with TNBC and low FOXP3 expression. Cox proportional multivariate analysis using all of the prognostic variables with p-values lower than 0.15 in the univariate analysis revealed that only a high number of FOXP3-positive Tregs in tumor cells (p = 0.049, HR 2.4 95% CI 1.0–5.6) was a significant independent prognostic factor for increased OS. For PFS, stage III and lymphovascular invasion, as well as a high number of FOXP3-positive Tregs, were statistically significant prognostic factors (p = 0.001, HR 0.3 95% CI 0.1–0.6; p = 0.002, HR 3.2 95% CI 1.5–6.5; p = 0.032, HR 2.0 95% CI 1.1–3.6, respectively) ().
Table II. Univariate analysis for metastatic overall survival and progression free survival to first line palliative chemotherapy in the 86 patients.
Figure 3. Kaplan-Meier Survival curves for PFS and OS. (A) showed PFS curves according to the status of positive FOXP3 cells. Analysis of PFS revealed a significantly better prognosis for FOXP3+ Tregs ≥ 15 compared with FOXP3+ Tregs < 15 (p = 0.036). (B) showed OS curves according to the status of positive FOXP3 cells. Analysis of OS revealed a significantly better prognosis for FOXP3+ Tregs ≥ 15 compared with FOXP3+ Tregs < 15 (p = 0.001).
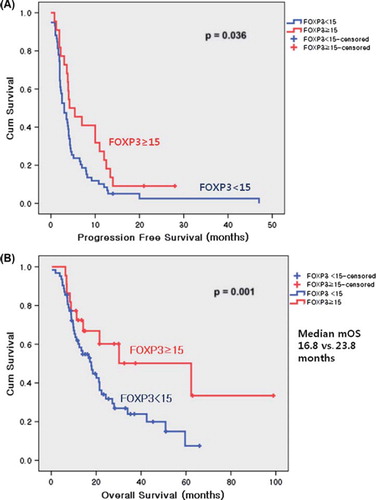
Table III. Multivariate proportional analysis of overall survival in TNBC patients.
Clinicopathologic characteristics of patients with metastatic OS ≥ 48 months
summarizes the characteristics of 11 patients with 48 months or longer metastatic OSs. Initial TNM staging and RFS did not appear to be characteristic features. However, 10 of the 11 patients had invasive ductal carcinoma and only one patient had metaplastic carcinoma. Interestingly, metastatic sites at initial metastasis were limited to distant lymph node, bone, and lungs. Excluding one patient, 10 patients showed high histologic grades. Accordingly, PFSs were longer and numbers of palliative chemotherapy lines were relatively higher than other patients. Obviously, numbers of FOXP3 positive cells were much higher than other population of patients (median 33).
Table IV. Clinicopathologic characteristics of the patients who had 48 months or longer metastatic overall survival.
Discussion
In this study, approximately 25.6% of triple-negative breast tumor specimens had FOXP3-positive Tregs. The goal of our study was to demonstrate significant relationships between the number of tumor-infiltrating Tregs and both clinicopathologic data and survival in TNBC patients. Tregs come in many forms with the most well-understood being those express CD4, CD25, and FOXP3. Among various subsets of Tregs, FOXP3 can be used as a good marker for CD4+CD25+ T cells as well as recent studies showing evidence for FOXP3 in CD4+CD25- T cells [Citation21].
One of the original and intriguing findings of this study was the observation that ≥ 15 FOXP3-positive and < 15 FOXP3-positive Tregs had opposing prognostic significance. There is highly significant association between FOXP3 expression and good prognosis as well as its independent prognostic value. This finding suggests that FOXP3 would have possibility as a new prognostic marker for breast carcinoma. However, the result needs to be validated and further study is warranted. Zuo et al. [Citation22] identified FOXP3 as a transcriptional repressor of the HER2/ERBB2 oncogene and the breast cancer oncogene SKP2. The authors showed that FOXP3 also suppressed growth and induced cell death in MCF-7 cells, a breast cancer line without HER2/ERBB2 overexpression [Citation23]. Functional studies of ≥ 15 FOXP3-positive and < 15 FOXP3-positive Tregs may shed more light on their role in the antitumor response and help to explain the observed associations with the prognosis.
A variety of prognostic and pathologic factors have been used to predict survival in breast cancer patients, including tumor size and grade, nodal status, and other biomarkers, such as ER, PR, and HER2. Although the current prognostic factors predict relapse in the first five years after therapy, it is unclear whether these parameters are useful in predicting long-term survival or late relapse, especially in patients with TNBC. According to Park et al. [Citation24], the AJCC TNM staging system does not work for TNBC patients, like as in other subtypes, and Kaplan-Meier curves of TNBC patients from stage I to IIIA show no significant difference in relapse free survival. The identity of favorable prognostic factors in TNBC remains a major unanswered question.
Curiel [Citation25] identified the inability of the immune system to eradicate established tumors as the seventh fundamental hallmark of cancer, which added to Hannahan and Weinberg's six fundamental hallmarks of cancer from 2000. However, the roles of immunosuppression in cancer evolution and treatment outcome are currently under evaluation [Citation26]. It has been hypothesized that immunologic factors, specifically Tregs, play a significant role in tumor development and progression due to their ability to induce immune tolerance to a cancer. It is now generally accepted that Tregs interfere with productive tumor immune surveillance and represent a major obstacle for the successful development of cancer immunotherapy. Tregs are generally considered to be immunosuppressive and have been linked to poor outcomes in several types of solid tumors [Citation3–14]. However, in the hormone-negative group of one study, there was no difference in survival between FOXP3-high and FOXP3-low patients [Citation27]. There is conflicting data in the literature about the role of Tregs in breast cancer patients. However, this study used FOXP3 mRNA expression using PCR [Citation27], not IHC. Probably, the difference in method might result in discrepancy of the result.
As one of the most aggressive breast cancer phenotypes, TNBC has been significantly associated with a higher average number of FOXP3-positive cells. Bohling et al. [Citation28] also noted a trend toward an increased number of FOXP3-positive cells and negative ER status. Recently, a few results were reported that Tregs including FOXP3 were associated with breast cancer pathogenesis and outcomes, especially for basal-like breast cancer [Citation29–31]. With respect to the mechanism, through which, FOXP3 expression might influence tumor aggressiveness, Hinz et al. [Citation32] have suggested that FOXP3 expression in pancreatic carcinoma cells may represent a type of molecular mimicry that enables immune evasion of tumor cells. These findings suggest that there may be a greater induction of immune tolerance to these more aggressive tumor types. Tregs in the tumor microenvironment are thought to function as mediators of immune evasion mechanisms.
Most interestingly, the patients who had 48 months or longer metastatic survival in our cohort showed unique clinicopathological features (). According to our analysis, there may be a few TNBC patients who show an indolent clinical course. Characteristic features of the patients showed high grade invasive ductal carcinoma at initial diagnosis. Most of them seemed to be responsive to adjuvant chemotherapy. However, their distant metastases developed independent of TNM tumor stages and metastatic sites usually limited to distant lymph nodes, bone, and lung. Their responses to palliative chemotherapy did not appear to be superior to those of other TNBCs, PFSs were relatively longer than other TNBC patients. Importantly, these sub- populations of TNBC patients would be strongly associated with increased FOXP3 Treg functions. Even though these findings were merely immature observations, it could be one plausible explanation for heterogeneity of TNBCs. It implies FOXP3 could be one of provocative biomarker in TNBCs. Although most of the studies for TNBC are mainly focused on BRCA1-deficient tumors, a recent study by comprehensive gene expression analysis showed TNBC could be divided into six subtypes [Citation32]. According to the study, BRCA-associated subtype is limited to 47% of all TNBC patients. In essence, there is immune modulatory subtype among several subtypes of TNBCs, in which immune signature is enriched for gene ontologies in immune cell process. Furthermore, the subtype overlaps with a gene signature for medullary breast cancer which is associated with a favorable prognosis despite its high-grade histology. In this regard, our study has significant implication for TNBC as described. Considering FOXP3 expression has favorable prognostication for TNBC in our study, FOXP3 may a candidate biomarker for immune modulatory subtype of TNBC.
In the clinical context, it is important to note that FOXP3 expression served to discriminate the prognostic risk of patients with TNBC, since OS of TNBC/≥ 15 FOXP3-positive patients was significantly better than that of TNBC/< 15 FOXP3-positive patients. These findings might aid in the identification of subgroups of patients who are more likely to have a good outcome and to whom specific therapies might be directed. It may offer a novel therapeutic target for these patients, who are not candidates for hormonal therapy or trastuzumab treatment.
Our study has several limitations, in which retrospective study usually have. First, the study population may have selection bias. In addition, heterogeneity of the treatment could affect the outcomes, which was not considered in this study. However, despite these main drawbacks, our study showed the possibility of Tregs as a biomarker for TNBC.
In conclusion, the present study is the first report on the prognostic significance of ≥ 15 FOXP3- positive and < 15 FOXP3-positive Tregs in TNBC. Although further studies are required before changes in clinical practice can be recommended, the present results suggest that assessment of ≥ 15 FOXP3-positive Tregs and < 15 FOXP3-positive Tregs in combination with other risk factors could improve the prognostic stratification of TNBC. Treg monitoring and manipulation in cancer patients has emerged as a promising new treatment option [Citation33].
Declaration of interest: This study was presented at the 2011 ASCO Annual Meeting as a poster presentation at June 6th 2011, Chicago, Illinois, USA. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, . Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429–34.
- Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, . Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006;295:2492–502.
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, . Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006;24:5373–80.
- Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, . FOXP3 expression and overall survival in breast cancer. J Clin Oncol 2009;27:1746–52.
- Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, . An evaluation of the clinical significance of FOXP3(+) infiltrating cells in human breast cancer. Breast Cancer Res Treat 2011;127:99–108.
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, . Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942–9.
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 2003;9:4404–8.
- Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, . CD4(+)CD25(+)CD127(low/-) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol 2009;131: 109–18.
- Yoshioka T, Miyamoto M, Cho Y, Ishikawa K, Tsuchikawa T, Kadoya M, . Infiltrating regulatory T cell numbers is not a factor to predict patient's survival in oesophageal squamous cell carcinoma. Br J Cancer 2008;98:1258–63.
- Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, . Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol 2009;27:186–92.
- Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, . Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 2004;173:1444–53.
- Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, . Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007;25:2586–93.
- Sasaki A, Tanaka F, Mimori K, Inoue H, Kai S, Shibata K, . Prognostic value of tumor-infiltrating FOXP3+ regulatory T cells in patients with hepatocellular carcinoma. Eur J Surg Oncol 2008;34:173–9.
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006;12:5423–34.
- Ladoire S, Arnould L, Mignot G, Coudert B, Rebe C, Chalmin F, . Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 2011;125:65–72.
- Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, . Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur J Immunol 2005;35:1681–91.
- Cho EY, Chang MH, Choi YL, Lee JE, Nam SJ, Yang JH, . Potential candidate biomarkers for heterogeneity in triple-negative breast cancer (TNBC). Cancer Chemother Pharmacol 2011;68:753–61.
- McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol 2005;2:416–22.
- Banham AH, Powrie FM, Suri-Payer E. FOXP3+ regulatory T cells: Current controversies and future perspectives. Eur J Immunol 2006;36:2832–6.
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol 2001;167:1245–53.
- Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, . Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest 2003;112:1437–43.
- Zuo T, Liu R, Zhang H, Chang X, Liu Y, Wang L, . FOXP3 is a novel transcriptional repressor for the breast cancer oncogene SKP2. J Clin Invest 2007;117:3765–73.
- Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, . FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell 2007;129:1275–86.
- Park YH, Lee SJ, Cho EY, Choi YL, Lee JE, Nam SJ, . Clinical relevance of TNM staging system according to breast cancer subtypes. Ann Oncol 2011;22:1554–60.
- Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest 2007;117:1167–74.
- Disis ML, Lyerly HK. Global role of the immune system in identifying cancer initiation and limiting disease progression. J Clin Oncol 2005;23:8923–5.
- Wolf AM, Rumpold H, Wolf D, Gastl G, Reimer D, Jenewein N, . Role of forkhead box protein 3 expression in invasive breast cancer. J Clin Oncol 2007;25:4499–500; author reply 500–1.
- Bohling SD, Allison KH. Immunosuppressive regulatory T cells are associated with aggressive breast cancer phenotypes: A potential therapeutic target. Mod Pathol 2008;21: 1527–32.
- Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, . CD8 cytotoxic T cell and FOXP3 regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat 2011;130:645–55.
- Wang J, Ray PS, Sim MS, Zhou XZ, Lu KP, Lee AV, . FOXC1 regulates the functions of human basal-like breast cancer cells by activating NF-kappaB signaling. Oncogene Epub 2012 Jan 16.
- Tkocz D, Crawford NT, Buckley NE, Berry FB, Kennedy RD, Gorski JJ, . BRCA1 and GATA3 corepress FOXC1 to inhibit the pathogenesis of basal-like breast cancers. Oncogene 2012;31:3667–78.
- Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, . Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res 2007;67:8344–50.
- Perez SA, Karamouzis MV, Skarlos DV, Ardavanis A, Sotiriadou NN, Iliopoulou EG, . CD4+CD25+ regulatory T-cell frequency in HER-2/neu (HER)-positive and HER-negative advanced-stage breast cancer patients. Clin Cancer Res 2007;13:2714–21.