Abstract
Background. More than 50% of breast cancer patients are diagnosed before the age of 65. Returning to work after treatment is, therefore, of interest for both the individual and society. The aim was to study the effect of support group intervention on sick leave and health care utilization in economic terms. Material and methods. Of 382 patients with newly diagnosed breast cancer, 191 + 191 patients were randomized to an intervention group or to a routine control group, respectively. The intervention group received support intervention on a residential basis for one week, followed by four days of follow-up two months later. The support intervention included informative-educational sections, relaxation training, mental visualization and non-verbal communication. Patients answered a questionnaire at baseline, two, six and 12 months about sick leave and health care utilization. Results. There was a trend towards longer sick leave and more health care utilization in the intervention group. The difference in total costs was statistically significantly higher in the intervention group after 12 months (p = 0.0036). Conclusion. Costs to society were not reduced with intervention in its present form.
Breast cancer is the most common malignant disease in women (excluding non-melanoma skin cancer). Annually 1.3 million women are diagnosed on a worldwide basis, while in Sweden 7300 women are diagnosed. The prognosis is generally good; approximately 90% are alive after five years, and 80% after 10 years. More than half of the women are diagnosed before the age of 65 and, thus, are in full-time work. In Sweden, more than 80% of women are employed. Returning to work after initial treatment is, therefore, in the interest of both the individual and society. For the individual woman, returning to work is a measure of normalization and recovery [Citation1]. For society, sick leave means loss of production and costs for health insurance. We know from an extensive meta-analysis that breast cancer survivors are more likely to be unemployed than healthy control participants [Citation2]. As reviewed by de Boer et al., proposed mechanisms are job discontinuation, difficulty combining treatment with full-time work and physical or mental limitations [Citation2]. Still, the majority of breast cancer survivors return to work. Bouknight [Citation3] found that more than 80% returned to work within 18 months, but obviously, some survivors do not [Citation4].
Several factors have been found to be associated with returning to work, such as chemotherapy [Citation5,Citation6], age [Citation7], education [Citation6,Citation8,Citation9] and income [Citation3], but very few randomized controlled studies have been carried out into interventions aimed at reducing the proportion of patients not returning to work [Citation10]. We have previously presented results from a prospective randomized controlled study of a support group intervention program, with the main objective of studying possible effects on mood, fatigue and quality of life [Citation11,Citation12]. A secondary aim of that study was to investigate possible effects on sick leave and health care utilization.
The aim of the present analysis was, within the framework of a prospective randomized controlled trial, to study the effect of support intervention, after breast cancer treatment, on sick leave, health care utilization and health economy in women with primary breast cancer. Our hypothesis was that the intervention would have a possible beneficial effect on the women’s symptoms, with less anxiety, depression and fatigue and better quality of life [Citation13,Citation14]. This effect would, in turn, lead to a shorter sick leave and a quicker return to work, with a corresponding reduction in the consumption of medical care and, finally, emanating in lower costs for society, however, this assumption has little evidence. Fors et al. [Citation15] had in their review intended to study work disability, however, they could not find any studies with these outcome measures.
Material and methods
Subjects
All women with a newly diagnosed primary breast cancer were, during their post-operative radiotherapy, considered for participation. They were included between April 2002 and November 2007 at the Department of Oncology at the Central Hospital in Västerås, Sweden. During this period, 770 patients were referred for radiotherapy and 709 were assessed for eligibility. Patients were, for logistical reasons, recruited during their treatment with radiotherapy. Most patients treated at the hospital were referred for radiotherapy, but, according to current regional guidelines, a few elderly women merely underwent a mastectomy [see flow chart ()]. The inclusion criteria in the study were a newly diagnosed primary breast cancer, no previous malignancy, the physical and mental capability to participate in group interventions and to fill in questionnaires and an expected survival time of more than 12 months. Due to the characteristics of the residential premises, patients with a physical disability were excluded. We also had to exclude patients with severe visual or hearing impairments, serious mental illness, dementia or active alcohol abuse, due to their inability to participate in the intervention. Patients who had participated in group rehabilitations were also excluded in total (54 patients). In the total group, the patients were between 30 and 84 years, which means that the issue of sick leave is not relevant to the entire group. We, therefore, limited the analyses to those who were under the age of 65 at the time of the intervention, which is the general age of retirement in Sweden.
Figure 1. Consort flow chart. Flow chart of participants’ progress through the randomized trial. CT, chemotherapy; RT, radiotherapy.
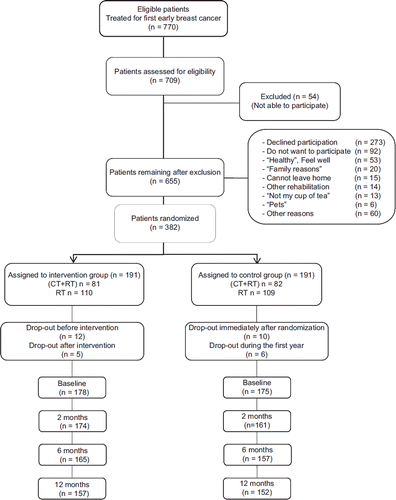
All those fulfilling the inclusion criteria were informed about the study and, after acceptance to participate, all patients provided their written informed consent. The Ethics Committee at the University of Uppsala approved the study and patients were treated according to the Declaration of Helsinki.
Patients were stratified according to adjuvant chemotherapy and randomized in blocks of four by the use of closed envelopes. In total, 382 women were included in the study, 191 in the intervention group and 191 in the control group.
Support intervention
The support-intervention program at the Foundation of Lustgården Mälardalen resort started in 1992 and was developed by discussions in the scientific community concerning the connections between emotions, immunity and malignant diseases. Professional persons, e.g. oncologists, surgeons, social workers, psychologists as well as patients were involved in the process that sought to identify what they thought would improve quality of life, or rather, what would meet the needs of the patients, which were not at that time met by ordinary clinical practice. The procedure was implemented in order to achieve a reasonable degree of face validity. This led to an information-based support program supplemented with relaxation, qi-gong and liberating dance. The intervention concept was fully developed and tested in a pilot [Citation16] study before the present study was initiated.
The intervention took place within four months of ending adjuvant treatment (chemotherapy and radiotherapy) and comprised a seven-day stay at the Foundation of Lustgården Mälardalen resort, where the participants took part in the support program, followed by a four-day follow-up two months after the initial visit. Trastuzumab and long-term endocrine treatment could be on-going.
Control patients were subjected to standard follow-up routines at the Department of Oncology or Surgery.
Questionnaires and analyses
Study patients answered questionnaires at baseline (after randomization but before intervention) as well as two, six and 12 months after the intervention. We used a questionnaire, that we formulated (see Supplementary Appendix Questionnaire, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.734921) with questions about family situation (single, married, cohabiting, divorced, children at home, etc.), and open questions about occupation, sick leave and health care utilization.
Sick leave. The questions explored whether the patient was currently on sick leave and to what extent, as well as how many days the patient had been on sick leave during the last 12 months. For calculations about the number of participants as well as the number of days on sick leave, we did not include women who at baseline stated that they were retirees, early retirees or had unpaid work. For the calculation of the cost of sick leave, we included all women who at the time of answering the questionnaire indicated that they had been on sick leave for at least one day during the last 12 months. Based on these self-reported data, we calculated an estimate of the costs for the sick leave period. Since no data on the participants’ actual incomes were available, it was decided that the costs for sick leave should be based on an average monthly income of 2900 EURO (25,000 SEK). According to the Swedish social security regulations during the years covered in the present study, this amounted to a sickness benefit of 73.62 EURO (631 SEK) per day. Most women were on sick leave during the intervention, which, in most cases occurred in close proximity to the treatment. However, we lack detailed information.
The Swedish regulation on sickness absence. The social insurance system in Sweden is publicly funded and covers all who reside or work in Sweden, providing financial protection for persons with a disability or in connection with an illness. The first 14 days of a sick leave period are paid by the employer. The sickness benefit is approximately 80% of the individual's income. You may be on the case of a sick leave for a maximum of 364 days during a 15-month period, but in severe disease such as cancer, this period can be extended. Disability pension can be granted if work capacity is permanently reduced by at least a quarter. One cannot, according to Swedish labour laws, be dismissed from work due to illness.
Health care utilization. Regarding health care utilization, the participants were asked separate questions about whether they had visited a general practitioner, a medical specialist, or a physiotherapist or performed any other health care visits. If the answer was affirmative, the participants were asked how many times they had visited each particular medical speciality during the last 12 months. In the calculations of health care consumption, we used the reported number of visits to any health care provider during the study period. For calculation of the costs for the different health care services, we used the calculated costs of a visit to a health care provider at the Västmanland's County Hospital, Västerås in the year 2005, which was 192 EURO for a doctor's appointment in primary care, 471 EURO for consultations by medical specialists and 87 EURO for visits to a physiotherapist (data from the financial unit at the hospital). However, we lack information about the cost of other health care visits and, therefore, this is not included in the economic calculation.
Total cost. The health economic cost was calculated separately as the sum of the cost of sick leave and the cost of health care utilization, with or without the cost of the intervention, 2300 EURO, which was the actual cost that we were charged, and included food, lodgings, personnel costs and other expenses for the boarding.
No calculation of the costs for society in terms of loss of production or filling of vacancies could be performed.
Power analysis
The present study is part of a randomized study covering different aspects of rehabilitation. Power calculation was performed based on the assumption that 50% of women treated for breast cancer show some sign of psychological distress [Citation17], which was reported in the literature at that time. To be able to detect a 15% lower proportion of psychological distress between the intervention and the control group after one year, with a power of 80% and a 5% significance level, we would need a total number of 340 patients. In order to allow for at least a 10% drop-out rate we aimed for 400 patients.
Statistical analysis
Differences between the intervention and control groups were tested with Pearson's χ2-test for categorical variables, except in one case when the assumptions behind Pearson's χ2-test were not fulfilled and Fisher's exact test had to be used instead. The Mann-Whitney test was used for discrete variables, and since not all continuous variables could be considered to be normally distributed, the Mann-Whitney test was used also for continuous variables. In the questionnaire, the number of days on sick leave during the last 12 months was categorized as 0, 1–2, 3–7, 8–14, 15–30, 31–60, 61–90, 91–180, 181–365 or > 365 days. For the analyses in this study, each category was replaced with the median of the category's lowest and highest values and > 365 days set to 365 days. The health economic cost of sick leave was then calculated as number of days on sick leave times 73.62 EURO. The number of days on sick leave as well as the health economic cost of sick leave were then treated as continuous variables. The number of visits to each specific kind of health care provider was categorized as 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, or > 10 visits. For the analyses in this study, the category > 10 visits was set to 11 visits. The economic cost of health care utilization was then calculated as the sum of: 1) the number of visits to a doctor in primary care 192 EURO (1374 SEK); 2) the number of visits for consultations by medical specialists 471 EURO (3360 SEK); and 3) the number of visits to a physiotherapist 87 EURO (620 SEK). The number of visits to each specific kind of health care provider was analyzed as a discrete variable, while the economic cost of health care utilization was treated as a continuous variable. Statistical analyses were performed with IBM SPSS Statistics and R, with p-values < 0.05 considered statistically significant.
Results
Primary treatment
Two hundred and ninety-three patients were treated with breast-conserving surgery and 89 underwent a mastectomy. One hundred and sixty underwent sentinel node biopsy only, 198 a level I–II axillary dissection and 24 patients had no axillary surgery. Chemotherapy was administered to 161 patients, either pre- or post-operatively. Radiotherapy was delivered to 375 patients – to the breast in all patients who had undergone breast-conserving operations and to adjacent lymph node stations if involvement of the axilla was present. Antibody treatment was used in patients with HER2-positive tumours and endocrine therapy to most endocrine responsive patients. For further details on primary treatment, see [Citation12]. The clinical characteristics of the patients were extracted from the patients’ records and are to be found in .
Table I. Distribution of patients according to surgical intervention, node status, tumor characteristics, menopausal status, post-operative endocrine treatment and civil status at baseline.
Response rate
The response rate was 92% at baseline, 88% at two months, 84% at six months and 81% at 12 months see flow chart ().
Sick leave
At baseline (time for randomization), 121 (63.4%) in the intervention and 115 (60.2%) in the control group (p = 0.528) were employable (defined as not retired, no early-age disability, and no unpaid employment). Of these, 20 were unemployed, 10 in the intervention group and 10 in the control group.
Figure 2. Proportion of women of working-age on sick leave, at baseline, 2, 6 and 12 months post-intervention. Women with retirement pension, disability pension and women with temporary disability are excluded.
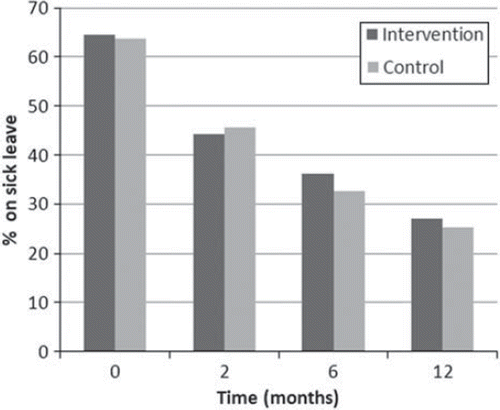
Of those that were employable at baseline, 71 (64.5%) in the intervention group and 65 (63.7%) in the control group were on sick leave (p = 0.901). At two, six and 12 months, 47 (44.3%) and 42 (45.7%) (p = 0.853), 38 (36.2%) and 29 (32.6%) (p = 0.599), 26 (27.1%) and 22 (25.3%) (p = 0.783) were on sick leave in the intervention and the control groups, respectively. The differences between the groups were, thus, not statistically significant ().
At baseline, women treated with chemotherapy in the intervention group had, on average, been on sick leave for 241 days during the previous 12 months compared with 234 in the control group. The accumulated sick leave for the previous 12-month period increased slightly in both the intervention and control group until the two month cut-off, but, thereafter, the proportion of women on sick leave decreased up to the 12-month follow-up in both groups. The differences between the groups were not statistically significant ().
Table II. Sick leave: Mean days on sick leave during the last 12 months following randomization in women of working-age. Retirees, early retirees or women with unpaid work are excluded. Comparison between intervention and control group, Patients are stratified according to treatment with chemotherapy.
Women not treated with chemotherapy in the intervention group, had on average only been on sick leave for 84 days compared with 86 days during the previous 12 months in the control group (p = 0.539). This increased slightly in the intervention group up to the six-month follow-up. In the control group, there was a decrease at two months and an increase at six months but a significant decrease in both groups up to 12 months. There was no significant difference between the groups at any point in time ().
Health care utilization
There was no statistically significant difference between the groups regarding the number of visits to medical specialists, general practitioners or physiotherapists at any time after the intervention period. There was no significant difference between the groups regarding contacts with other health care providers (e.g. chiropractors, natrapaths and masseurs) at baseline or at two months but, of those treated with chemotherapy, women in the intervention group consulted other health care providers more often than women in the control group after six and 12 months (p = 0.006 and p = 0.015, respectively) ().
Table III. Health care utilization: Average number of visits to general practitioners, hospital specialist, physiotherapists and other health care providers for the 12 months following randomization. Intervention group vs. control group at baseline, 2, 6 and 12 months after randomization. Patients are stratified according to treatment.
Health economics
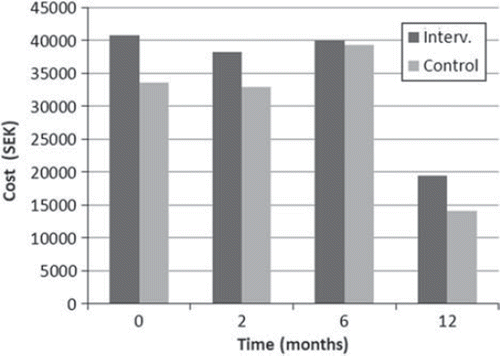
The total costs for sick leave and consumption of health services at each follow-up during the study period decreased in both the intervention and control group from baseline to the 12- month follow-up. The total costs for the intervention group were higher at all points in time and the differences between the groups reached statistical significance after 12 months (Mann-Whitney p = 0.036) (, ).
Table IV. Total cost of sick leave and health care utilization (SEK) for the 12 months following randomization. Intervention group compared with control group.
Figure 3. Total cost of sick leave and health care utilization (SEK) for the 12 months following randomization for the whole study population. Cost of intervention not included.
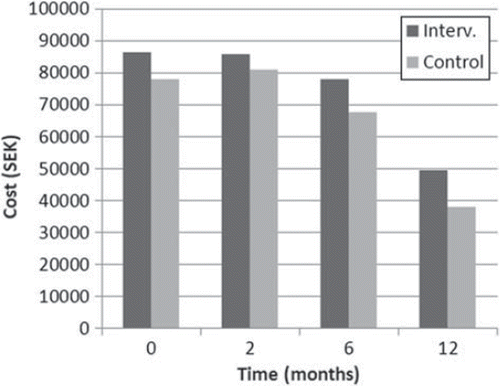
Figure 4. Total cost of sick leave and health care utilization during the last 12 months for the 12 months following randomization for women treated with chemotherapy. Cost of intervention not included.
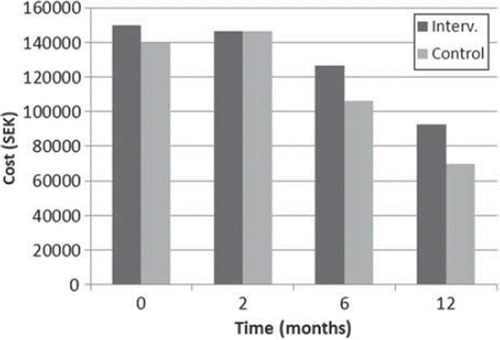
Figure 5. Total cost of sick leave and health care utilization for the 12 months following randomization at each measuring point for women not treated with chemotherapy. Cost of intervention not included.
Adding the cost of the intervention made the cost for the intervention group statistically significantly higher at all times of measurement.
Discussion
This prospective randomized trial of the effects of support intervention in women with primary breast cancer showed no positive effects of the intervention on sick leave, health care utilization and health economy. On the contrary, there was a tendency for women undergoing the intervention to have a longer sick-leave period and to seek other health care providers more often when compared with control patients. The total cost of sick leave and consumption of health services was statistically significantly higher for the intervention group after 12 months, and it was also higher, but not significantly higher at any measured time, even before the cost of the intervention has been included. This is contrary to the results of Simpson et al. [Citation18], who found a 23.5% cost reduction after a psycho-social intervention, but they only studied the effect of the intervention on the cost of health care consumption. Analyses of whether positive effects on anxiety [Citation12] or other psychosocial or existential effects outweigh the increased costs were beyond the scope of this article.
The reasons for the lack of a positive effect could be multifactorial. The intervention could have been too short to alleviate patients’ symptoms [Citation19], or the content of the intervention might have been suboptimal to demonstrate any direct impact on sick leave and health care consumption. There exists only a few previous studies for comparison, since intervention studies rarely measured sick leave or had return to work as an outcome [Citation10]. A recently published Cochrane review demonstrated low quality evidence for psychological interventions on return to work rates and a moderate quality evidence for multidisciplinary interventions involving physical, psychological and vocational components [Citation20]. Another important aspect may be that the intervention actually influenced the patients’ thoughts and feelings and created a need for sick leave to handle and cope with their anxiety. This was also discussed in an article by Damjaer [Citation21] et al. where they studied early retirement after breast cancer. During the intervention, many women had the opportunity to focus on themselves for the first time in their life and not take care of family and relatives. This may have led to a change of priorities in favor of a longer sick leave [Citation7].
Another weakness in our study may be that women themselves had to state their sick leave and we did not collect the data from any official records. On the other hand, the women in this study were asked to indicate the extent to which they had been on sick leave due to their breast cancer. This distinction might be difficult to disentangle from a register. Women may, however, have interpreted this question differently. Some may have regarded the whole sick leave period as caused by breast cancer and treatment while others may have interpreted it as fatigue or depression. This distinction is probably difficult to disentangle even if you study the sick leave records since the same may apply to doctors issuing the certificates. One could, therefore, argue that any sick leave period during the follow-up should be regarded as caused by breast cancer.
Another weakness is that we lack information about the income of the women and, therefore, had to estimate this as the average income in Sweden. It is well known that breast cancer is more frequent in women from higher social classes. However, for comparison, of the two randomized groups the exact level of income does not matter. We also lack data on women's health before the breast cancer diagnosis, which can also be regarded as failing. Petersson [Citation22] et al. showed that women with poorer health before diagnosis had longer sick- leave periods. The study participants had, during the intervention, received an opportunity to try different methods of alleviating symptoms, such as massage, relaxation and qigong. This may have contributed to women in the intervention group who received chemotherapy to search for other health care providers such as massage therapists.
Bouknight [Citation3] showed that work-place adjustments played an important role in breast cancer patients’ return to work, which has also been shown by Pryce [Citation23] in a study of patients with different cancer diagnoses. Perhaps this is a better way forward, having a multimodal approach and working closely with employers, when planning for patients’ return to work. Previous studies have put forward the idea that interventions to help patients return to work should be individually tailored and conducted in close co-operation with occupational health experts and employers [Citation24,Citation25] and studies on this are ongoing and should be investigated further.
Since we could not see any faster return to work or reduced number of physician and physiotherapist visits in the intervention group, we were not able to show any economic gain from this type of this intervention, rather a higher cost. The question is, of course, whether other types of intervention with more physical elements would be more cost-effective but Haines [Citation26] et al. could not show any efficacy and economic efficiency of a multimodal physical activity program. In a study by Lemieux [Citation27] on psychosocial intervention in metastatic breast cancer patients, they could not show any decrease in health care system resource utilization.
The cost of our intervention was, when we conducted our study, 2300 EURO per patient and, thus the economic net effect of this type of rehabilitation is negative.
Conclusion
We conclude that we could not show any positive economic effect of support intervention on sick leave and, health care in this setting, with residential intervention for one week and four days of follow-up. In fact we saw a tendency towards longer sick leave and more health care utilization and we could show that the total cost in the intervention group was actually higher and that this difference was statistically significant after 12 months. Future randomized studies with sick leave as outcome measures, should be work-directed, in closer co-operation with employers and insurance agencies, to make it easier for cancer patients to return to work.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.734921
Download PDF (213.5 KB)Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was funded by grants from the County Council of Västmanland, the Swedish Social Insurance Agency, the Västmanland Research Fund against Cancer and the National Federation of Cancer and Traffic Injury.
References
- Spelten ER, Verbeek JH, Uitterhoeve AL, Ansink AC, van der Lelie J, de Reijke TM, . Cancer, fatigue and the return of patients to work – a prospective cohort study. Eur J Cancer 2003;39:1562–7.
- de Boer AG, Taskila T, Ojajarvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: A meta-analysis and meta-regression. JAMA 2009;301:753–62.
- Bouknight RR, Bradley CJ, Luo Z. Correlates of return to work for breast cancer survivors. J Clin Oncol 2006; 24:345–53.
- Roelen CA, Koopmans PC, van Rhenen W, Groothoff JW, van der Klink JJ, Bultmann U. Trends in return to work of breast cancer survivors. Breast Cancer Res Treat 2011;128:237–42.
- Johnsson A, Fornander T, Rutqvist LE, Olsson M. Factors influencing return to work: A narrative study of women treated for breast cancer. Eur J Cancer Care (Engl) 2010;19:317–23.
- Eaker S, Wigertz A, Lambert PC, Bergkvist L, Ahlgren J, Lambe M. Breast cancer, sickness absence, income and marital status. A study on life situation 1 year prior diagnosis compared to 3 and 5 years after diagnosis. PLoS One 2011;6:e18040.
- Drolet M, Maunsell E, Brisson J, Brisson C, Masse B, Deschenes L. Not working 3 years after breast cancer: Predictors in a population-based study. J Clin Oncol 2005; 23:8305–12.
- Spelten ER, Sprangers MA, Verbeek JH. Factors reported to influence the return to work of cancer survivors: A literature review. Psychooncology 2002;11:124–31.
- Taskila T, Lindbohm ML. Factors affecting cancer survivors’ employment and work ability. Acta Oncol 2007;46:446–51.
- Hoving JL, Broekhuizen ML, Frings-Dresen MH. Return to work of breast cancer survivors: A systematic review of intervention studies. BMC Cancer 2009;9:117.
- Bjorneklett HG, Lindemalm C, Ojutkangas ML, Berglund A, Letocha H, Strang P, . A randomized controlled trial of a support group intervention on the quality of life and fatigue in women after primary treatment for early breast cancer. Support Care Cancer Epub 2012 May 11.
- Bjorneklett HG, Lindemalm C, Rosenblad A, Ojutkangas ML, Letocha H, Strang P, . A randomised controlled trial of support group intervention after breast cancer treatment: Results on anxiety and depression. Acta Oncol 2012;51: 198–207.
- Arndt V, Stegmaier C, Ziegler H, Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer 2006;107:2496–503.
- Visser MR, Smets EM. Fatigue, depression and quality of life in cancer patients: How are they related?Support Care Cancer 1998;6:101–8.
- Fors EA, Bertheussen GF, Thune I, Juvet LK, Elvsaas IK, Oldervoll L, . Psychosocial interventions as part of breast cancer rehabilitation programs? Results from a systematic review. Psychooncology 2011;20:909–18.
- Lindemalm C, Strang P, Lekander M. Support group for cancer patients. Does it improve their physical and psychological wellbeing? A pilot study. Support Care Cancer 2005; 13:652–7.
- Burgess C, Cornelius V, Love S, Graham J, Richards M, Ramirez A. Depression and anxiety in women with early breast cancer: Five year observational cohort study. Br Med J 2005;330:702.
- Simpson JS, Carlson LE, Trew ME. Effect of group therapy for breast cancer on healthcare utilization. Cancer Pract 2001;9:19–26.
- Rehse B, Pukrop R. Effects of psychosocial interventions on quality of life in adult cancer patients: Meta analysis of 37 published controlled outcome studies. Patient Educ Couns 2003;50:179–86.
- de Boer AG, Taskila T, Tamminga SJ, Frings-Dresen MH, Feuerstein M, Verbeek JH. Interventions to enhance return-to-work for cancer patients. Cochrane Database Syst Rev 2011;2:CD007569.
- Damkjae LH, Deltour I, Suppli NP, Christensen J, Kroman NT, Johansen C, . Breast cancer and early retirement: Associations with disease characteristics, treatment, comorbidity, social position and participation in a six-day rehabilitation course in a register-based study in Denmark. Acta Oncol 2011;50:274–81.
- Petersson LM, Wennman-Larsen A, Nilsson M, Olsson M, Alexanderson K. Work situation and sickness absence in the initial period after breast cancer surgery. Acta Oncol 2011; 50:282–8.
- Pryce J, Munir F, Haslam C. Cancer survivorship and work: Symptoms, supervisor response, co-worker disclosure and work adjustment. J Occup Rehabil 2007;17:83–92.
- Tamminga SJ, de Boer AG, Verbeek JH, Taskila T, Frings-Dresen MH. Enhancing return-to-work in cancer patients, development of an intervention and design of a randomised controlled trial. BMC Cancer 2010;10:345.
- Tamminga SJ, de Boer AG, Verbeek JH, Frings-Dresen MH. Return-to-work interventions integrated into cancer care: A systematic review. Occup Environ Med 2010;67:639–48.
- Haines TP, Sinnamon P, Wetzig NG, Lehman M, Walpole E, Pratt T, . Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res Treat 2010;124:163–75.
- Lemieux J, Topp A, Chappell H, Ennis M, Goodwin PJ. Economic analysis of psychosocial group therapy in women with metastatic breast cancer. Breast Cancer Res Treat 2006;100:183–90.