Abstract
Background. Successful cell therapy relies on the identification and mass expansion of functional cells for infusion. Cryopreservation of cells is an inevitable step in most cell therapies which also entails consequences for the frozen cells. Material and methods. This study assessed the impact of cryopreservation and the widely used protocol for rapid expansion of T lymphocytes. The effects on cell viability, immunocompetence and the impact on apoptotic and immunosuppressive marker expression were analyzed using validated assays. Results and conclusion. Cryopreservation of lymphocytes during the rapid expansion protocol did not affect cell viability. Lymphocytes that underwent mass expansion or culture in high dose IL-2 were unable to respond to PHA stimulation by intracellular ATP production immediately after thawing (ATP = 16 ± 11 ng/ml). However, their reactivity to PHA was regained within 48 hours of recovery (ATP = 356 ± 61 ng/ml). Analysis of mRNA levels revealed downregulation of TGF-β and IL-10 at all time points. Culture in high dose IL-2 led to upregulation of p73 and BCL-2 mRNA levels while FoxP3 expression was elevated after culture in IL-2 and artificial TCR stimuli. FoxP3 levels decreased after short-term recovery without IL-2 or stimulation. Antigen specificity, as determined by IFNγ secretion, was unaffected by cryopreservation but was completely lost after addition of high dose IL-2 and artificial TCR stimuli. In conclusion, allowing short-time recovery of mass expanded and cryopreserved cells before reinfusion could enhance the outcome of adoptive cell therapy as the cells regain immune competence and specificity.
Adoptive cell therapy (ACT) is an encouraging approach for treatment of malignant hematological and solid tumors. ACT in tumor immunotherapy is based on infusion of high numbers of tumor antigen-specific T cells isolated from peripheral blood, draining lymph nodes or tumor tissues [Citation1]. These cells are characterized, mass-expanded and re-infused to the lymphodepleted autologous host [Citation2]. Successful therapy relies on treatment with viable and avid cells which in turn directly depend on correct handling of large cell batches. In ACT, cryopreservation of immune cells is a standard procedure that is routinely performed in order to preserve cells for future use. It also allows for the establishment of specimen banks for future analysis while reducing problems such as assay variabilities that are common when independently isolated fresh samples are analyzed. Large-scale cell expansions for ACT may involve up to two freeze/thawing steps. The first is usually performed after isolation and characterization of optimal cells for therapy and the second may be performed after mass expansion prior to infusion. Although cryopreservation is a valuable tool, it is also a rather harsh treatment of the cells with the potential to induce considerable alterations in cell functions. Significant cell loss due to cryopreservation caused by formation of intracellular ice crystals and disruption of cell membrane during freezing has been observed [Citation3]. It is suggested that although storage at low temperature blocks most enzymatic pathways and metabolism in the cells, some of the apoptotic mechanisms caused by physiological signals still remain active and cause apoptosis or senescence [Citation4]. There are reports on loss of response and reduced T cell functions in frozen cells compared to freshly isolated cells and loss of antigen recognition of T cells that increases with prolongation of freezing time [Citation5,Citation6]. Freezing and thawing of the cells may also cause phenotypic alterations such as loss of cell surface receptors and change in cytokine production by peripheral blood mononuclear cells (PBMCs) [Citation7,Citation8].
In order to obtain therapeutically sufficient numbers, the T cells are usually cultured with high dose interleukin 2 (IL-2) together with polyclonal activation of the T cell receptor (TCR) with anti-CD3 antibodies. Several reports describe induction of apoptosis involving p73 and Fas pathways following prolonged TCR ligation [Citation9,Citation10]. In addition, several groups have reported the induction of FoxP3 expression and an increase in numbers of regulatory T cells after culture in high dose IL-2 combined with TCR stimuli [Citation11]. Given that prolonged TCR activation and expansion of lymphocytes may result in activation-induced cell death (AICD), senescence and expansion of possible regulatory T cell subsets, it is crucial to assess the quality of the manufactured cells prior to ACT [Citation12].
This study was designed to determine the effects of rapid expansion and cryopreservation on lymphocytes at different time points during the course of the procedure. We tested fresh versus frozen/thawed samples from the same donors with polyclonal stimuli to assess immune responsiveness in vitro. Moreover, we performed a comparative analysis evaluating the expression of markers for immunosuppression and apoptosis at the mRNA level at different time points throughout the protocol. Finally we compared the effects of cryopreservation and rapid expansion on antigen specificity and reactivity of fresh and frozen/thawed CMV-specific lymphocytes by measuring IFNγ secretion in response to stimulation with a CMV pp65-derived peptide. To our knowledge, this is the first study where the combined effects of rapid expansion and cryopreservation of T cells have been explored.
Material and methods
Study design
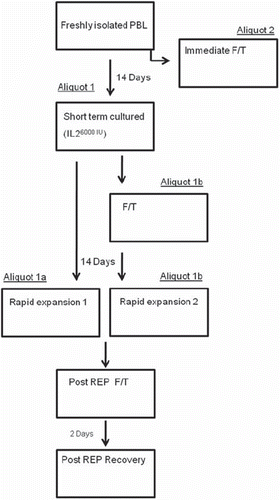
Lymphocytes were isolated from PBMCs from five healthy donors. These cells were used to simulate the effects of cryopreservation and thawing of tumor infiltrating lymphocytes (TILs) during the process of rapid expansion. In order to imitate the settings of TIL isolation and rapid expansion, freshly isolated lymphocytes were cultured with high concentration of interleukin (IL)-2 for a period of 14 days where after the cells were expanded according to the rapid expansion protocol (REP) developed at the NIH. During this period the vitality of cells was monitored using the Cylex Immuknow® test. This test was also performed on cryopreserved TILs isolated from tumor biopsies obtained from six melanoma patients. We quantified the levels of mRNA encoding pro- or anti-apoptotic markers and inhibitory molecules. This was done with high reproducibility and accuracy with real-time quantitative PCR and the levels of markers in the lymphocytes were compared after cryopreservation and during cultivation and expansion. From each donor, freshly isolated lymphocytes were divided into two aliquots (1 and 2): aliquot 1 was cultured for 14 days in 6000 IU/ml IL-2 (Novartis, Basel, Switzerland) and aliquot 2 was cryopreserved and thawed after three days for analyses. Aliquot 1 was further divided in two new aliquots (aliquot 1a and 1b): aliquot 1a was immediately expanded according to the rapid expansion protocol (REP) and aliquot 1b was first cryopreserved and thawed for analyses where after it was expanded according to REP. After a period of 14 days of expansion, aliquots 1a and 1b were cryopreserved, thawed and analyzed. Upon thawing, a portion of cells from both aliquots were allowed to recover for a period of 48 hours in complete media without addition of IL-2 and then analyzed. The study design is summarized in .
Figure 1. The progress flow sheet for the treatment of cells displayed in sequential steps. Isolated lymphocytes from five donors were split into two aliquots. Aliquot 2 was immediately cryopreserved and thawed. Aliquot 1 was cultured for 2 weeks and split into 1a and 1b. Aliquot 1a was expanded and cryopreserved, while aliquot 1b was cryopreserved before expansion. Both aliquots 1a and 1b were cryopreserved after expansion and were allowed to recover for 48 hours after thawing. During the progression of the treatment schedule, each box, representing different time points were analyzed.
Ethical approval, patients and samples
Melanoma biopsies were collected within an ethical committee, approved protocol (reference number 2005:383).
Isolation of tumor-infiltrating lymphocytes
TIL cultures were prepared as previously described in detail [Citation13]. Briefly, multiple independent TIL cultures were initiated in 2 ml wells containing tumor fragments (1 mm3) in complete medium (CM) consisting of RPMI1640 with 12 mM glutamine, 10% heat-inactivated human AB serum, 25 mM HEPES, 10 ug/ml Penicillin-Streptomycin (PEST) and 5.5 × 10−5 M 2-mercaptoethanol. 6000 IU/ml of IL-2 (Novartis, Basel, Switzerland) was added to each well. Half of the media and IL-2 were changed on day 5 and thereafter every third day after initiation. About 2–3 × 106 TILs were harvested after 12–15 days when confluent growth in each original well was observed. By pooling the wells, approximately 50 × 106 TILs were obtained. Pooled TILs were immediately cryopreserved for future use.
Isolation of peripheral blood mononuclear cells (PBMCs)
Buffy coats from standard whole blood units were obtained from five healthy blood donors. PBMCs were isolated by Ficoll-Paque (Amersham Biosciences, Uppsala, Sweden) gradient centrifugation. The lymphocyte fractions were separated by plastic adherence, for 90 minutes at 37°C in T-75 culture flasks (Corning, NY, USA). Lymphocytes were collected as non-adherent cells and were cultured in complete medium (CM) (RPMI1640, supplemented with 10% AB serum, 1% Penicillin, Streptomycin (PEST), 1% HEPES, 0.5% 1 mM L-Glutamine and 0.2% 20 μM 2-mercaptoethanol). All cell culture reagents were purchased from Invitrogen (Carlsbad, CA, USA).
Rapid expansion of peripheral blood lymphocytes (PBLs)
Expansion of PBLs was performed using the REP [Citation2,Citation14]. Briefly, cells were co-cultured with 200-fold excess of irradiated (55 Gy) allogeneic PBMCs from at least five healthy donors as feeder cells in standing T75 flasks. Cells were cultured in standing T-75 flasks in CM containing 5% human AB serum, 30 ng/ml agonistic anti-CD3 antibody (Ortho Biotech, Bridgewater, NJ, USA) and 600 IU/ml IL-2. Half of the media was changed on day 5 using CM and 600 IU/ml IL-2 and cells were transferred to standing T-175 flasks.
Cryopreservation and thawing of cells
Freezing solution (85% complement inactivated AB serum and 15% dimethyl sulfoxide (DMSO, Apoteket, Sweden). Cells were preserved at 107 cell/ml in cryogenic vials (Nalgene Labware, Nalge Company, Rochester, NY, USA) and stored for 24 hours at −80°C in Nalgene Cryo freezing containers (Nalge Nunc International, Rochester, NY, USA). Frozen cells were thereafter transferred to a gaseous nitrogen freezer for cryopreservation. Frozen cells were thawed in 37°C water bath until approximately 4/5 of the freezing solution was thawed. The cells were resuspended in complete medium and washed twice. Frozen/thawed cells were counted and assessed for viability using trypan blue exclusion.
Cylex Immuknow® immune function test
Immuknow® is a test for monitoring of T cell immune responses in transplanted patients. In this test lymphocytes are stimulated with phytohemagglutinin (PHA), a non-specific mitogen which stimulates T cells regardless of their antigen specificity or phenotypic status. The amount of produced intracellular adenosine triphosphate (ATP) is then measured as an indicator of cellular activation. The immune function assay was performed with minor modifications to the manufacturer’s protocol. Briefly, lymphocytes were diluted with a sample diluent, added to a microtiter plate well, and incubated with PHA for 15 to 18 hours in a 37°C, 5% CO2 incubator. The following day, CD4 + cells were positively selected within the microwells with magnetic particles coated with anti-human CD4 monoclonal antibodies (Dynabeads, Dynal, Oslo, Norway) and a strong magnet (model 1050 magnet tray, Cylex, Inc., Columbia, MD, USA), washed to remove residual cells, and lysed to release intracellular ATP. Released ATP was measured with a luciferin/luciferase system and a luminometer (Berthold, Knoxville, TN, USA). In this assay 1 × 106 PBMCs or PBLs were diluted 1:4 with the sample diluent. Magnetic separation approximately isolated about 3–5 × 105 CD4 +cells. According to the manufacturer, the limit of ATP detection in this assay is 1 ng/ml.
Quantitative Real-time PCR (qRT-PCR)
Quantitative RT-PCR was performed to determine the gene expression levels of pro/anti-apoptotic and immunosuppressive markers. The panel of analyzed genes consisted of markers for: immunoregulation (IL-10, FoxP3, TGF-β), apoptosis (p73, FasL and BCL-2) and β-actin as housekeeping gene. Primer oligo sequences (), validated for specificity and efficacy were retrieved from the Harvard Primer Bank and purchased from Sigma. Validated FOXP3 primers were obtained from Qiagen. Total RNA was extracted from lymphocytes using the RNeasy kit (Qiagen, CA, USA). Reverse transcription reaction with 1 μg of total RNA in 100 μl was carried out using the SuperScript II Reverse Transcription Kit following the manufacturer´s instructions (Invitrogen). Quantitative PCR was performed in 25 μl reaction volumes using, 200 nM of primers and the Power SYBR Green Master Mix (Applied Biosystems). All reactions were performed on the 7500 Real-Time PCR system (Applied Biosystems).
Table I. Primers used in RT-qPCR gene expression analysis.
Target gene mRNA levels were normalized to β-actin mRNA according to the following formula: [2^−(CT target − CT actin)]× 100%, where CT is the threshold cycle. Fold change was calculated by dividing the normalized target gene expression of manipulated cells by that of the freshly isolated cells.
Flow cytometric analysis
Cells were suspended in PBS supplemented with 1% human serum albumin. Cells were stained with surface antibodies APC-conjugated anti CD3 and PE-conjugated anti CD4 (Becton Dickinson, San Jose, CA, USA) for 30 minutes at 4°C in the dark. The cells were washed twice in PBS before fixation. Intercellular staining was performed with FITC- conjugated anti human FoxP3 (PCH101) and control isotype mAb (eBiosciences, San Diego, CA, USA). Data acquisition and analysis was performed using the FACSCalibur and the CellQuest Pro software (Becton Dickinson).
CMV ELISpot
Buffy coats were obtained from 13 healthy CMV seropositive blood donors. Three donors with a high proportion of CD8+ T cells recognizing the HLA-A*0201 restricted CMV derived pp65495-503 peptide were identified using tetramer staining. Briefly, 200 μl of buffy coat was lysed using BD FACS lysing solution (BD) according to manufacturers’ guidelines. White blood cells were subsequently stained with CD8-PerCP, CD3-APC (BD) and PE-labeled HLA-A*0201/pp65495-503 tetramer (Beckman-Coulter, San Diego, CA, USA). PBMCs were obtained from these donors using Ficoll Paque (GE Healthcare, Uppsala, Sweden) gradient centrifugation according to manufacturers’ instructions and washed three times in PBS. Cells were subjected to analysis of IFNγ production in response to stimulation with the CMV derived pp65495-503 peptide using ELISPOT, either directly after isolation or after cryopreservation and REP.
A MultiScreen® filter plate [(MAIPSWU10), Millipore, Billerica, MA USA] was coated one day prior to cell isolation with an IFNγ capturing antibody (Mabtech, Nacka, Sweden) as per manufacturer and incubated over night at 4°C. Cells were seeded at a density of 1.5 × 105 cells per well in triplicates, in CM and incubated with 5 μg/well of the pp65495-503 (NLVPMVATV) peptide (Genscript, Piscataway, NJ, USA) overnight. The irrelevant VMAT-131-39 (LLLDNMLFT) derived peptide (Sigma Genosys, Haverhill, Suffolk, UK) and medium alone were used as negative controls. Subsequent washing steps, antibody incubations and spot development were performed according to the manufacturers’ instructions (Mabtech). Spot forming cells were counted using the AID ELISpot reader (Autoimmun Diagnostika GmbH, Strassberg, Germany) and data analysis was performed using the Immunospot® software (CTL, Bonn, Germany). A significant difference in spot formation after CMV peptide stimulation compared to spot formation in response to irrelevant peptide and no peptide controls was considered a specific response. Statistically significant differences were calculated using one way ANOVA and Bonferroni´s multiple comparison test. A p-value of < 0.05 was considered significant.
Statistical analysis
Analysis was performed using GraphPad prism software version 5.01 (La Jolla, CA, USA), using the two-tailed Wilcoxon matched pair test. Statistical analyses for ELISpot were performed using one way ANOVA test and Bonferroni´s multiple comparison test. Values of p < 0.05 were considered to be statistically significant.
Results
Cell viability after freeze/thawing
Cell viability was > 80% after each thawing when assessed with trypan blue exclusion. There was about 10–15% cell loss during each round of cryopreservation ().
Table II. Cell viability.
Effects of cryopreservation on production of intracellular ATP
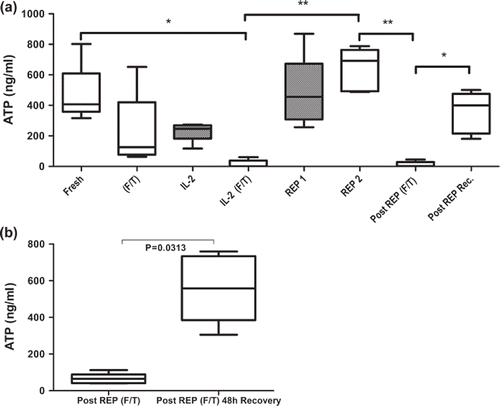
Cells were monitored with the Immuknow® immune function test prior to and after each freeze/thawing step during the rapid expansion protocol. Based on the Immuknow® assay CD4+ T cells from freshly isolated PBMC showed moderate responses to PHA stimulation corresponding to 468 ± 85 ng/ml of intracellular ATP (a). After 24 hours of cryopreservation and subsequent thawing the ATP level was reduced to 224 ± 109 ng/ml (aliquot 2). After 14 days of culture with high dose IL-2, the ATP level was reduced to 229 ± 28 ng/ml (aliquot 1a) and after cryopreservation and thawing ATP levels were significantly reduced to 16 ± 11 ng/ml (aliquot 1b). Both cell populations (aliquot 1a and 1b) were expanded according to the rapid expansion protocol. After 14 days of rapid expansion, cells subjected to prior cryopreservation (aliquot 1b) showed significantly increased ATP levels (641 ± 62 ng/ml). Cryopreservation and thawing of those cells reduced the ATP levels to 12 ± 8 ng/ml. The cells were allowed to recover for 48 hours after the final cryopreservation which resulted in increased ATP to 356 ± 61 ng/ml. We observed similar results when we measured the ATP content of cryopreserved TILs isolated from six melanoma patients. When allowed to recover for 48 hours after cryopreservation, the intercellular ATP content of the TILs increased from 67 ± 28 ng/ml to 552 ± 188 ng/ml (b).
Figure 2. Concentration of intercellular ATP concentration as a marker for metabolic activity and immunocompetence of CD4+ T cell subsets isolated from peripheral blood of healthy donors (a) and TILs isolated from melanoma biopsies (b) was measured with the Immuknow assay. Cells were stimulated with PHA for 24 hours and lysed to release intracellular ATP. Released ATP was measured with a luciferin/luciferase system and a luminometer. (a) Figure showing changes in ATP concentration throughout the protocol of; fresh cells isolated from healthy blood donors (Fresh); cryopreserved and thawed cells (F/T); non-cryopreserved cells cultured for 14 days in high-dose IL-2 (IL-2); cells cultured for 14 days in high-dose IL-2 and then cryopreserved and thawed (IL-2 F/T); non-cryopreserved and cryopreserved day 14 cells after rapid expansion (REP1 and REP2, respectively); REP2 cells after cryopreservation and thawing (Post REP F/T) and REP2 cells allowed to recover for 48 hours after cryopreservation and thawing (Post REP Rec.). F/T (frozen/thawed), gray bars represent non-cryopreserved cells. (b) Immuknow results from cryopreserved TILs from melanoma patients. TILs obtained from six melanoma patients were stimulated with PHA directly after thawing and after 48 hours of recovery. The boxes represent concentrations of intracellular ATP with median, minimum and maximum values (n = 5). Asterisks denote statistical significance where *p ≤ 0.05, **p ≤ 0.01.
Quantitative Real-time PCR
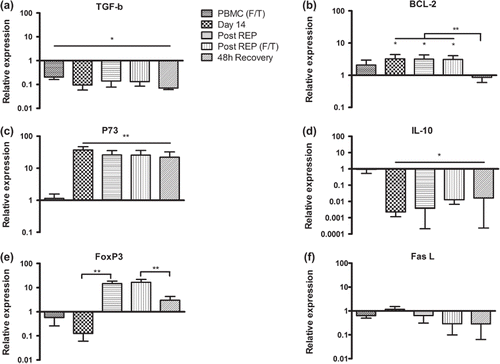
Cryopreservation of fresh PBMC did result in an approximately 10-fold decrease in the mRNA levels of TGF-β which remained stable throughout the protocol (a). The levels of Bcl-2 were not affected by immediate freeze thawing, but increased by approximately five-fold after 14 days of culture in IL-2 and remained elevated to the time point after rapid expansion. The levels were significantly decreased after 48 hours of recovery and were similar to those of fresh PBMC (b). After 14 days of culture in high dose IL-2 there was a 30-fold increase of p73 mRNA levels and a 30-fold decrease of IL-10 compared to fresh cells (c and d). These alterations remained throughout the protocol. We observed an approximately 10-fold decrease of FoxP3 mRNA levels after 14 days of culture in high dose IL-2. However, after rapid expansion, FoxP3 levels increased by 25-fold but declined to approximately four-fold over baseline when the cells were allowed to recover in IL-2 free medium (e). This correlated with intracellular staining for FOXP3 in the same cell populations (data not shown). The expression of FasL mRNA was not significantly changed at any point during the protocol (f). Cryopreservation prior to rapid expansion, as described for aliquot 1b, had no significant effect on mRNA levels of the analyzed markers (not shown).
Figure 3. Relative expression levels of (a) TGF-β, (b) Bcl-2, (c) p73, (d) IL-10, (e) FoxP3 and (f) FasL in PBMCs from healthy donors at different time points during the protocol. All values were normalized to the expression of β-actin and gene expression in fresh PBMC was set as one. F/T – frozen/thawed. Bars represent mean fold change (n = 5) + SD. Asterisks denote statistical significance where *p ≤ 0.05, **p ≤ 0.01.
ELISpot
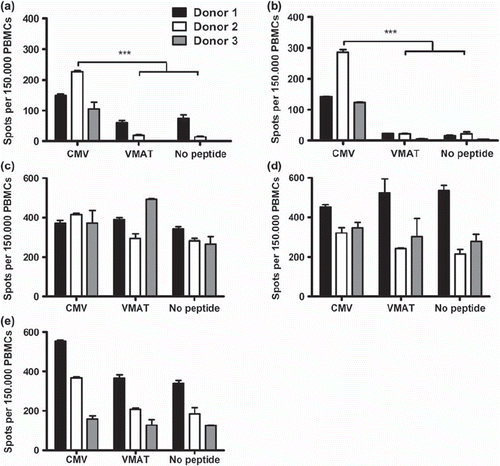
To detect any effects of cryopreservation and rapid expansion on antigen-specific CD8+ T cell responses, a series of ELISpot assays were performed. PBMCs from three CMV seropositive donors were stimulated with a HLA-A0201-restricted, CMV pp65-derived peptide directly after Ficoll separation, cryopreservation, culture in high dose IL-2 for 14 days and rapid expansion. Cells stimulated directly after Ficoll separation generated a significantly higher number of IFNγ spots in response to the pp65 peptide compared to control peptide stimulation and no peptide controls, which confirmed their CMV specificity (a). Cryopreservation did not impact the antigen specific reactivity as all donors showed significantly higher reactivity for the pp65 peptide compared to controls when analyzed after thawing and immediate stimulation (b). After two weeks of culture in high dose IL-2, specific IFNγ secretion after peptide stimulation was no longer detected (c). After completion of the rapid expansion, background IFNγ secretion was still high in all donors and no specific responses were observed (d). After 48 hours of culture in the absence of IL-2 there was a clear, although not significant (p˜0.06), restoration of antigenic specificity manifested by an overall reduction of background IFNγ secretion and specific responses were detected in two of three donors (e).
Figure 4. ELISpot assays showing the number of spot-forming cells in response to stimulation with CMV pp65 and unspecific peptide VMAT-1 in healthy CMV+ donors. Cells stimulated directly after isolation (a), immediately after freeze/thawing (b), after 14 days of culture in high dose IL-2 (c), after rapid expansion (d) and after 48 hours of recovery without IL-2 (e). Numbers of spot-forming cells per 1.5 × 105 cells were measured. Bars represent mean of triplicate wells and SD. Asterisks denote statistical significance where *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001. One of two representative experiments is shown.
Discussion
In this study we investigated the impact of rapid expansion and cryopreservation on lymphocytes isolated from peripheral blood of healthy donors. Cryopreservation of activated and stimulated T cells did not, at any step during the protocol, lead to a significant decrease in cell viability. After the third cycle of cryopreservation, we observed the same amount of cell loss as in the first step. Next, we measured the amount of ATP production in response to PHA stimulation, comparing fresh and cryopreserved T cells. Using the Immuknow assay we observed that cryopreservation has a profound effect on the ATP production of T cells. Cryopreserved cells did not respond to PHA stimulation and displayed significantly lower concentrations of intracellular ATP when compared to fresh cells. ATP concentrations returned to normal levels when the cells were allowed to recover for 48 hours or when cryopreserved cells were rapidly expanded. T cells cultured for two weeks in high dose IL-2 did also displayed a significant reduction in intracellular ATP concentrations as compared to fresh T cells. ATP levels were however normalized after rapid expansion regardless of cryopreservation. Importantly, the results derived from patient TILs were comparable in terms of ATP concentration. In addition to cell viability and immune-competence, possible alterations in apoptotic and immunosuppressive marker expression during rapid expansion and cryopreservation were investigated. Cryopreservation alone reduced the relative expression of TGF-β in cryopreserved cells when compared to freshly isolated PBMC. The same reduction in TGF-β as well as IL-10 levels could also be observed after 14 days of culture in high dose IL-2 and remained reduced during the rest of the protocol. FoxP3 expression increased by 20-fold after rapid expansion. Given the simultaneous decrease in immunosuppressive TGF-β and IL-10 mRNA levels, the rise in FoxP3 expression could be interpreted as a marker for activation of T cells rather than induction or proliferation of preexisting Treg. IL-2 and TCR stimuli have been shown to transiently upregulate FoxP3 expression in T cells. These cells lack the regulatory properties of natural Tregs and do not suppress proliferation or cytokine production through secretion of, e.g. TGF-β and IL-10 [Citation15]. The FoxP3 levels were reduced by 50% after 48 hours recovery in the absence of IL-2 and CD3 stimuli which further supports that FoxP3 is transiently upregulated upon stimulation and should not be interpreted as a marker for Tregs.
The mRNA levels of p73 increased after addition of IL-2. The protein p73 was originally described as a mediator of apoptosis [Citation16] and increase of p73 could be an indicator of TCR-activation-induced cell death (TCR-AICD) that occurs with strong stimulation of TCR [Citation10]. In this study we observed that expanded cells were non-apoptotic despite increased levels of p73 mRNA. This could be explained by the fact that our qPCR analysis did not discriminate between the many variants of p73 isoforms, of which some have anti-apoptotic properties. It is well established that isoforms with alternative N-terminals have the ability to inhibit Fas and Bax mediated apoptosis [Citation17]. The unchanged expression of FasL indicated the absence of Fas-mediated activation-induced apoptosis, which is a known mechanism of clonal deletion of T cells [Citation18]. Overall, the gene expression profile indicates that rapid polyclonal expansion and cryopreservation do not cause apoptosis or induction of regulatory cell populations.
In contrast to stimulated ATP production which was heavily impaired, cryopreservation did not significantly alter the ability of CMV pp65-specific T cells to secrete IFNγ in response to cognate antigen stimulation as revealed by ELISpot analysis. After two weeks of culture in high dose IL-2, unspecific IFNγ secretion was dramatically increased in all donors and specific responses to antigen could no longer be detected. Background IFNγ secretion was still high after rapid expansion; however, a specific response was again detected in one donor. The high background responses corroborate a previously published report where high-scale expansion of TILs with polyclonal stimulus resulted in a decrease in the percentage of tumor-reactive T cells [Citation19]. The high background responses are probably a consequence of IL-2 addition as it is known to induce IFNγ production in an unspecific manner [Citation20]. The relative contribution of unspecific IFNγ secretion comparing CD8+/CD4+ was not completely elucidated due to lack of cells. Short-time recovery of expanded cells resulted in a decrease in background IFNγ secretion and distinct antigen-specific responses were again detected in two donors. It is likely that an extended recovery period could further decrease the background levels. The optimal culture time for rejuvenation of T cell metabolic competence and antigenic specificity needs to be elucidated in upcoming studies.
Our findings may be of importance for the improvement of T cell therapy as they implicate that allowing short-time recovery of mass expanded and cryopreserved cells before reinfusion could enhance the outcome of adoptive cell therapy as the cells regain immune competence and specificity.
Acknowledgments
We thank Raja Choudhury and Kajsa Lundberg at Cancer Centre Karolinska, Karolinska Institute, for help with acquisition and analysis of ELISpot data. We would also like to thank Gabriella Paul-Wetterberg for assistance in the qPCR analysis.
Declaration of interest: This study was supported by grants from the Swedish Cancer Society, the Swedish Research Council and an Uppsala University Hospital ALF grant. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. All authors have declared that there is no financial conflict of interest in regards to this work.
References
- Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008;8:299–308.
- Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother 2003;26:332–42.
- Jeurink PV, Vissers YM, Rappard B, Savelkoul HF. T cell responses in fresh and cryopreserved peripheral blood mononuclear cells: Kinetics of cell viability, cellular subsets, proliferation, and cytokine production. Cryobiology 2008;57: 91–103.
- Fowke KR, Behnke J, Hanson C, Shea K, Cosentino LM. Apoptosis: A method for evaluating the cryopreservation of whole blood and peripheral blood mononuclear cells. J Immunol Methods 2000;244:139–44.
- Costantini A, Mancini S, Giuliodoro S, Butini L, Regnery CM, Silvestri G, et al. Effects of cryopreservation on lymphocyte immunophenotype and function. J Immunol Methods 2003;278:145–55.
- Owen RE, Sinclair E, Emu B, Heitman JW, Hirschkorn DF, Epling CL, et al. Loss of T cell responses following long-term cryopreservation. J Immunol Methods 2007;326:93–115.
- Tollerud DJ, Brown LM, Clark JW, Neuland CY, Mann DL, Pankiw-Trost LK, et al. Cryopreservation and long-term liquid nitrogen storage of peripheral blood mononuclear cells for flow cytometry analysis: Effects on cell subset proportions and fluorescence intensity. J Clin Lab Anal 1991;5:255–61.
- Axelsson S, Faresjo M, Hedman M, Ludvigsson J, Casas R. Cryopreserved peripheral blood mononuclear cells are suitable for the assessment of immunological markers in type 1 diabetic children. Cryobiology 2008;57:201–8.
- Schmitz I, Krueger A, Baumann S, Schulze-Bergkamen H, Krammer PH, Kirchhoff S. An IL-2-dependent switch between CD95 signaling pathways sensitizes primary human T cells toward CD95-mediated activation-induced cell death. J Immunol 2003;171:2930–6.
- Lissy NA, Davis PK, Irwin M, Kaelin WG, Dowdy SF. A common E2F-1 and p73 pathway mediates cell death induced by TCR activation. Nature 2000;407:642–5.
- Ahmadzadeh M, Antony PA, Rosenberg SA. IL-2 and IL-15 each mediate de novo induction of FOXP3 expression in human tumor antigen-specific CD8 T cells. J Immunother 2007;30:294–302.
- Krammer PH. CD95’s deadly mission in the immune system. Nature 2000;407:789–95.
- Carlsson B, Sadeghi A, Bengtsson M, Wagenius G, Totterman TH. Effector T cell analysis of melanoma tumor-infiltrating lymphocyte cultures using HLA-ABC semimatched melanoma cell lines. J Immunother 2008;31: 633–43.
- Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods 1990;128: 189–201.
- Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4 + T cells. Eur J Immunol 2007;37: 129–38.
- Jost CA, Marin MC, Kaelin WG, Jr.p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature 1997;389:191–4.
- Muller M, Schilling T, Sayan AE, Kairat A, Lorenz K, Schulze-Bergkamen H, et al. TAp73/Delta Np73 influences apoptotic response, chemosensitivity and prognosis in hepatocellular carcinoma. Cell Death Differ 2005;12:1564–77.
- Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, et al. Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 1995;373:441–4.
- Pandolfino MC, Labarriere N, Tessier MH, Cassidanius A, Bercegeay S, Lemarre P, et al. High-scale expansion of melanoma-reactive TIL by a polyclonal stimulus: Predictability and relation with disease advancement. Cancer Immunol Immunother 2001;50:134–40.
- Bream JH, Hodge DL, Gonsky R, Spolski R, Leonard WJ, Krebs S, et al. A distal region in the interferon-gamma gene is a site of epigenetic remodeling and transcriptional regulation by interleukin-2. J Biol Chem 2004;279:41249–57.