Abstract
Background. Patients with head and neck squamous cell carcinoma (HNSCC) often lose a considerable amount of muscle mass following the disease and treatment. This is an independent mortality predictor, lowering muscle strength and functional performance. Progressive resistance training (PRT) increases muscle mass among healthy individuals and groups of cancer patients, but it has not been investigated in HNSCC patients. Furthermore, studies in healthy subjects show an additive effect of protein and creatine supplementation following PRT. Objectives. Firstly, to investigate the feasibility of 12 weeks of PRT ± protein and creatine supplementation among HNSCC patients. Secondly, to investigate group changes over time and group differences regarding lean body mass (LBM), muscle strength and functional performance following PRT ± dietary supplementation. Material and methods. Thirty patients were randomized into two groups: a PROCR group undergoing a seven-day pre-trial creatine loading protocol followed by 12 weeks of PRT with creatine and protein supplementation and a PLA group undergoing a seven-day pre-trial placebo ingestion protocol followed by an identical PRT protocol with placebo supplementation. Before the pre-trial and pre- and post-PRT evaluation of LBM, maximal isometric and isokinetic muscle strength and functional performance were performed. Results. Seventy percent of the patients completed the intervention and the PRT adherence rate was 97%. No significant group differences were found in any endpoints. From pre- to post-PRT, LBM increased significantly in the PROCR group by 2.6 ± 2.2 kg (p < 0.0001) and increased in the PLA group (1.3 ± 1.1 kg, p = 0.07). Maximal isometric and isokinetic muscle strength as well as functional performance increased significantly in both groups. Conclusion. PRT is feasible in radiotherapy treated HNSCC patients. Following PRT, lean body mass, muscle strength and functional performance increased significantly in both groups (LBM only borderline significant in PLA group) with no significant group difference in any endpoint.
The majority of radiotherapy treated head and neck squamous cell carcinoma (HNSCC) patients experience a weight loss which averages 6–12% of pre-treatment body weight [Citation1]. This weight loss may be caused by the cancer disease per se (cachexia) and/or predominantly as a side effect from radiation and/or chemotherapy. Side effects such as mucositis, changes in saliva viscosity, xerostomia and dysphagia impede sufficient energy intake causing weight loss, which may persist more than two years post-treatment [Citation2,Citation3].
Weight loss negatively impacts survival, morbidity and functional performance in HNSCC patients [Citation4,Citation5]. Silver and colleagues [Citation1] reported a mean weight loss of 11 kg one month after radiotherapy treatment of which 72% was lean body mass (LBM). This was associated with a decline in physical performance and an increase in functional dependence. Further, they observed decline in daily physical activity level [Citation1]. Likewise, Jager-Wittenaar et al. [Citation6] found a 4.5% treatment induced decrease in LBM in HNSCC patients coupled with a significant loss of muscle strength. Consequently, interventions to effectively regain muscle mass and physical function in survivors of HNSCC are needed.
A growing number of studies show that exercise in various cancer patients is safe and improves physical fitness, muscle strength and functional performance [Citation7–10]. Specifically, progressive resistance training (PRT) increases muscle mass, strength and functional performance. This is shown in healthy individuals and elderly with muscle wasting (i.e. age-related sarcopenia) [Citation11]. Studies on the effects of PRT in cancer survivors are limited and primarily involve breast- and prostate cancer survivors [Citation8]. Moreover, few studies have focused on changes in LBM in cancer survivors [Citation9,Citation12].
One randomized controlled trial applying PRT in HNSCC patients with shoulder dysfunction after neck dissection (no radiotherapy) showed that 12 weeks of upper extremity PRT improved muscle strength and endurance and reduced shoulder pain and disability [Citation13]. However, this study did not evaluate whole body LBM and functional performance.
In young healthy individuals the effects of PRT on muscle mass, strength and physical performance can be improved by timed protein ingestion [Citation14]. In elderly persons, however, an additive effect of protein supplementation is less clear. On the one hand, Esmarck et al. [Citation15] showed a superior effect on skeletal muscle hypertrophy in elderly males after 12 weeks of PRT with protein ingestion just after training compared to males ingesting protein 2 h post-training. On the other hand, Verdijk et al. [Citation16] showed no additive effect of timed protein ingestion compared to placebo following 12 weeks of PRT in elderly men. To our knowledge no studies on PRT combined with protein supplementation in cancer patients are published.
Creatine is another ergogenic supplement, which has been shown to induce an additive effect on increases in LBM, muscle strength and functional performance in young people [Citation17], but with equivocal effects in the aging population with some studies indicating a benefit from creatine ingestion and some studies showing no additive effect above PRT alone [Citation18].
The primary purpose of this study was to investigate the feasibility of whole body PRT ± protein and creatine supplementation. Secondly, to investigate group changes over time and group differences regarding LBM, muscle strength and functional performance. We hypothesized, that PRT is feasible and that LBM, muscle strength and functional performance increase in both groups with an additive effect on LBM and muscle strength from PRT with protein and creatine supplementation.
Material and methods
Setting and patients
The present study was a single center, randomized, stratified and parallel-grouped feasibility study. Approval was received from the local ethics committee for the Central Denmark Region (journal number: 20090181), and the study was registered at clinicaltrials.gov (Identifier: NCT01025518).
Eligible participants fulfilled the following inclusion criteria: 1) Histologically diagnosed with squamous cell carcinoma of the larynx (except glottic stage I + II), pharynx, oral cavity or in lymph nodes from an unknown primary tumor [stage I–IV and tumor node metastasis (TNM) classification (AJCC, 2002)]; 2) Terminated curative radiotherapy treatment ± chemotherapy; 3) Complete tumor remission and no metastases; 4) No current or previous malignancies, psychological, social or geographical conditions that could prevent participation, testing or training; 5) No excessive alcohol intake (men > 21 and women > 14 units/wk); 6) WHO performance status of 0–1; 7) Age > 18 years; and 8) written consent.
Radiotherapy
All patients received primary radiotherapy given with curative intent according to the DAHANCA guidelines (www.dahanca.dk). All 30 patients received accelerated radiotherapy with IMRT to total tumor dose of 66–68 Gy, with 6 fractions per week, and with a total dose of 50 Gy to the elective neck volume. The radiotherapy was in all patients combined with the hypoxic cell sensitizer Nimorazole (1200 mg/m2), and in addition, 20 patients received weekly concomitant Cisplatin (40 mg/m²). Furthermore, four patients received the EGFr-antibody Zalutumumab. No elective neck dissection was conducted except for two patients with metastasis from an unknown primary tumor.
At the two months post-treatment follow-up, patients were given oral and written information about participation before signing a written consent. Before randomization patients were stratified according to HPV status (positive vs. negative), relative weight loss (cut-off: 8.5% of pre-treatment body weight) and feeding tube vs. no feeding tube during treatment. Patients were randomly allocated to either group 1 (PROCR, n = 16) or group 2 (PLA, n = 14).
Progressive resistance training and dietary supplementation
Following pre-trial supplementation, where PROCR ingested creatine and PLA ingested placebo supplement for seven days (see Supplementary Appendix, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.741325), all patients began a 12-week PRT protocol, which consisted of 30 training sessions evenly dispersed over 12 weeks at local commercial training facilities. Apart from two or three instruction sessions during the first two weeks and further on average five supervisions during the remaining 10 weeks, all other training sessions were unsupervised. Subjects performed a whole body training program consisting of seven conventional exercises (leg press, knee extension, hamstring curls, chest press, sit ups, back extensions and lateral pull down) involving the largest muscle groups of the body.
On training days, PROCR patients ingested both creatine (5 g) and protein powder (30 g) supplementation and PLA patients ingested isocaloric placebo (maltodextrine) in close relation to every training session. On non-training days the PROCR group followed a creatine maintaining protocol and the PLA group ingested placebo (see Supplementary Appendix, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.741325).
Feasibility outcomes
The feasibility outcomes were inclusion rate defined as the number of patients recruited from the number of eligible patients and completion rate defined as the number of participants able to complete the intervention and all three testing sessions. Adherence to the exercise intervention was evaluated via patient-reported training logs also providing information on individual progression in training frequency, volume and load. Adherence to the dietary supplementation was reported by means of questionnaires administered after pre-trial and 12 weeks of training. Any adverse events during the entire intervention period were recorded.
Endpoint evaluation and efficacy outcomes
All endpoints were evaluated at baseline, after the pre-trial supplementation, and following 12 weeks of PRT. Due to the prolonged physical testing, patients did not undergo sampling and testing in the fasting state, but were encouraged to eat and drink a standardized diet before all three testing sessions. All assessors involved in testing and data collection were blinded regarding group assignment of patients.
Primary outcome (LBM)
Whole body LBM and fat mass (FM) was determined using dual energy x-ray absorptiometry (DEXA) with narrow fan beam technology (Lunar Prodigy Advance, GE Healthcare Technologies, Madison, WI, USA). The DEXA scans were analyzed using Prodigy enCORE software. Patients were scanned clothed from head to toe in the supine position. The mean coefficient of variation of total body LBM and FM were 0.37% and 0.67%, respectively, determined from duplicate scans of three individuals.
Muscle strength
Maximal voluntary knee extensor (KE) and flexor (KF) strength (peak torque, Newton- meters) were determined using isokinetic dynamometry. For each patient maximal isokinetic (60°/s) and isometric KE (70° knee joint angle) and KF (20° knee joint angle) strength were determined (see Supplementary Appendix, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.741325).
Functional performance
Maximal walking performance was measured during a 10 m walk test; sit to stand performance was measured by the 30 s maximal sit-to-stand test; maximal stair climbing performance was measured as the shortest time to ascend two flights of stairs; arm function was evaluated by the 30 s maximal arm curl test (lifting a 3 kg and 4 kg dumbbell for women and men, respectively).
Between training sessions 20–26 all patients were asked to do a four-day diet registration to retrieve information on total energy, protein, fat and carbohydrate intake during the training period. Patients were asked to weigh all solid and liquid foods on three weekdays and one weekend day within one week.
Sample size
Based on a power calculation with an expected difference of 5% in LBM change between groups as reported previously [Citation15], we randomized 30 patients. SD was set to 4%. The calculation was based on an anticipated drop-out rate of 20%, power was 0.8 and level of significance was 0.05 and was performed in STATA ver. 11.2. (StataCorp, LT, Texas, USA).
Statistics
All data followed a normal distribution (tested using box plots, q-q-plots, histograms and dot-plots). Multivariate analyses of variance (MANOVA) were used to evaluate possible time and group interactions on all endpoints. In case of any group-time interactions or time effects within groups a post hoc multilevel mixed-effects linear regression analysis was done. All endpoints were tested statistically using a 5% level of significance. All outcomes were analyzed as per protocol, because of the feasibility purpose. Unless otherwise stated, data are presented as mean values ± SD. All analyses were performed using STATA version 11.2.
Results
Flow of patients
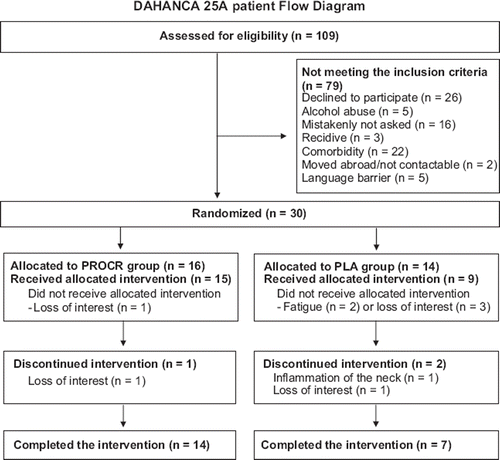
Between June 2010 and June 2011, there were 109 eligible HNSCC patients, of which 30 were randomized, giving a recruitment rate of 28%. All included patients completed the treatment according to the DAHANCA guidelines. The main reasons for not being enrolled were refusal, comorbidity (primarily psychological or musculo-skeletal problems hindering completion of the PRT protocol) and mistakenly not asked (). All patients were randomized within four weeks of the two months check-up.
The completion rate was 70% (n = 21). Of the nine non-completers (two patients from the PROCR group and seven from the PLA group), five dropped out prior to baseline testing because of change of mind after being randomized. Of the last four drop outs, one dropped out just after baseline testing (loss of interest), whereas three dropped out during the intervention due to loss of motivation (n = 2) and long-term illness due to radiation induced neck inflammation (n = 1).
presents baseline patient characteristics for the two groups. The mean weight loss from pre-treatment to the two month check-up was 9.2 ± 4.9 kg (range 2.9–21.0) kg in the PROCR group and 6.9 ± 4.4 (range 0.1–12.9) kg in the PLA group.
Table I. Characteristics of included patients and patients who completed the intervention in the two groups.
Feasibility outcomes
The mean training adherence rate was 97% with the completers participating in 29 (range 23–30) of the planned 30 training sessions. Reasons for not completing all sessions included vacation, illness or non-reported reasons. The mean completion time for the planned training sessions was 13 weeks (range 12–20). No major adverse events or unexpected side effects (injuries or health problems) from the training or supplementation were reported in any of the participants. Four subjects reported paused progression in one or two training exercises due to overuse symptoms in knees or shoulders.
During the 12-week training period 76% (n = 16) of all patients gave information on compliance to the dietary supplementation. Of these, 69% (n = 11) ingested all supplementation, 19% (n = 3) missed ≤ 3 supplementations, whereas 12% (n = 2, both in the PROCR group) terminated the supplementation four weeks prematurely due to muscle cramping (4–5 times weekly) and increased mucus production.
Body composition
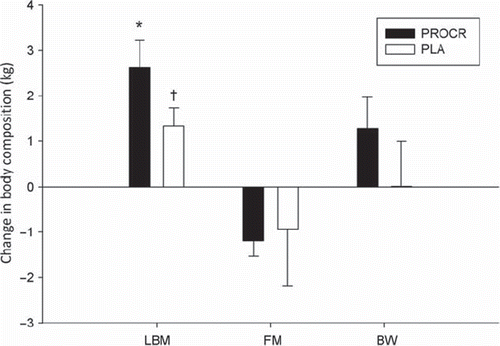
As shown in in the PROCR group, LBM increased significantly by 5.0 ± 3.8% from 50.5 ± 9.3 to 53.1 ± 10.8 kg (p < 0.0001) during the 12-week training intervention and in the PLA group 12 weeks of training induced an increase of 2.8 ± 2.5% from 50.4 ± 8.4 to 51.7 ± 8.3 kg (p = 0.07). There were no differences in BW, LBM or FM between groups at any time. During the PRT BW increased non-significantly from 70.3 ± 10.6 to 71.5 ± 11.8 kg in PROCR and was unchanged before and after PRT in PLA at 68.1 ± 12.0 and 68.1 ± 11.1 kg. At the same time FM decreased non-significantly from 16.7 ± 8.9 to 15.6 ± 9.0 kg in PROCR and from 14.6 ± 7.3 to 13.6 ± 6.6 kg in PLA.
Figure 2. Change in body composition (kg) following 12 weeks of PRT. *p < 0.05. †p = 0.07. Values presented as mean change ± SEM.
Subsequent analyses showed a trend towards a superior training effect on LBM when related to HPV status. Thus, across groups only 16% (1 of 6) of the HPV negative patients had a LBM increase larger than the mean increase of both groups combined (which was 4.2%), whereas 53% (8 of 15) of the HPV positive patients increased LBM at or above the mean value. The mean LBM increase in the HPV positive patients was 2.7 ± 2.0 kg which was 1.8 kg larger (p = 0.06; 95% CI 20.04; 3.7; linear regression analysis) than the HPV negative patients, who increased their LBM by 0.9 ± 1.2 kg. There was no difference in LBM increase following PRT between chemoradiation treated patients and patients treated with radiation therapy alone.
Muscle strength and functional performance
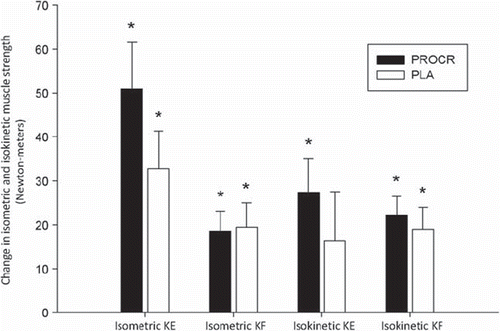
In both groups isometric and isokinetic muscle strength increased significantly from pre to post-PRT with no significant differences between groups (). (See supplementary Appendix for specific values on muscle strength, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.741325).
Figure 3. Change in isometric and isokinetic muscle strength following 12 weeks of PRT (Newton-meters). *p < 0.05. Values presented as mean change ± SEM. KE: knee extension, KF: knee flexion.
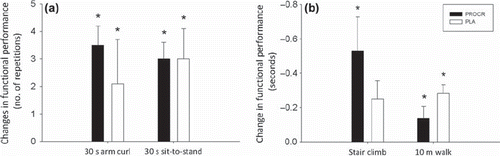
At baseline, there was no difference between groups in any of the functional performance tests. After PRT similar significant improvements in functional tests were seen in the two groups (). (see Supplementary Appendix for specific values for all functional tests, available online at http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.741325).
Discussion
The main finding of the present study is that PRT is feasible in radiotherapy treated HNSCC patients. LBM, muscle strength and functional performance increased significantly over time in both groups (LBM only borderline significant in PLA group) following PRT with no significant group difference in any endpoint.
Feasibility
The PRT adherence rate was very high (97%) in this study considering the community-based training modality with minimal supervision. This is noteworthy since this modality of training may represent a more realistic approach towards HNSCC rehabilitation than supervised training due to the lower financial costs and higher degree of flexibility for the patients. Adherence was similar to that of McNeely and colleges [Citation13], who reported an adherence rate of 95% during 12 weeks of supervised upper body PRT in HNSCC patients. Exercise studies on mixed cancer survivors with similar training duration have primarily involved completely supervised training sessions and report adherence rates ranging from 67% to 97%. [Citation19] Probably, a high motivation following a tough but successful treatment and the flexibility of our study design to reschedule missed training sessions contributed to the high adherence rate. Furthermore, no serious adverse events or side effects were registered further supporting the feasibility of PRT in radiotherapy treated HNSCC patients.
A completion rate of 70% was observed. Of the nine non-completers, six participants regretted their consent and dropped out already prior to the start of the training intervention (five prior to baseline testing) due to loss of interest and treatment related fatigue. In total there were seven drop outs in the PLA group compared to only two in the PROCR group indicating a systematical influence on adherence. Patients were blinded to group allocation and furthermore patients of both groups ingested the same volume of supplementation at the same time points with no difference in time and effort to participate. Moreover, as shown in the flow diagram, five of seven drop outs of the PLA group occurred prior to the intervention, emphasizing that the intervention per se did not influence their decision. In total, only three (10%) dropped out during the PRT period supporting the feasibility of PRT in radiotherapy treated HNSCC patients. Similarly, McNeely et al. report completion rates of 85% and 88% [Citation13,Citation20] after 12 weeks of supervised upper body PRT in HNSCC patients.
Changes in body composition
To our knowledge, this is the first study to report LBM changes after PRT in radiotherapy treated HNSCC patients. We observed significant increases in PROCR and PLA by 2.6 kg and 1.3 kg (non-significantly in PLA), respectively. This is a greater increase than previous reports showing increases of 0.5–0.9 kg in studies on breast cancer survivors undergoing 8–26 weeks of PRT [Citation21,Citation22]. Studies in healthy elderly people report LBM increases of around 2 kg following PRT [Citation11]. In particular, a comparable study by Eliot et al. [Citation23] reported that LBM increased by 1.6 kg following 14 weeks of PRT with protein and creatine supplementation in a healthy population aged 42–78 years. In addition, we consider the increase in LBM of this study clinically relevant, since this corresponds to the 2.4 kg LBM loss reported in HNSCC patients after treatment by Jager-Wittenaar and colleagues [Citation6]. Despite the smaller treatment induced weight loss found in their study, it seems that a large proportion of LBM loss can be reversed through 12 weeks PRT.
The 1.3 kg group difference in mean LBM change in the PLA group compared to the PROCR group did not reach significance indicating no clear additive effect of protein and creatine supplementation. The additive effect of protein and creatine supplementation in the elderly population remains equivocal [Citation18]. Thus, Eliot et al. [Citation23] failed to show an additive effect on changes in LBM of protein and/or creatine supplementation compared with placebo supplementation in elderly men undergoing PRT. We observed, however, a numerically two-fold difference in LBM increase between the PROCR and PLA groups and although this difference was not significant, it should be taken into consideration that the power of the analysis is low because of a large dropout rate of the PLA group potentially leading to a type II error. If real, the group difference could be clinically relevant and would speak in favor of supplementation.
According to the diet registration, the mean dietary protein ingestion of the participants in our study was 1.3 ± 0.4 and 1.4 ± 0.5 g/kg BW/day in the PROCR and PLA group, respectively, with no difference between groups despite the protein supplementation in the PROCR group. To increase protein synthesis and build LBM in the elderly, a minimum protein intake of 1.0 g/kg/BW/day together with PRT has been suggested [Citation24]. Consequently, both groups likely ingested sufficient dietary protein to fully support training induced increases in LBM, which could explain why the timed protein-creatine ingestion offered no further advantage.
Interestingly, the effect of PRT on LBM change seems to be superior in HPV positive patients, compared to HPV negative patients. This is in line with the better prognosis of survival of HPV positive patients and the significantly lower co-morbidity in this subgroup of patients [Citation25], which may influence positively on the feasibility of PRT in HNSCC patients. This is supported by the fact that 50% (6/12) of the HPV negative patients did not complete this study, whereas only 17% (3/18) of the HPV positive patients dropped out. These observations could be clinically relevant, but we conclude cautiously due to the small sample size. Total BW remained unchanged in both groups despite the LBM increase because of a simultaneous albeit non-significant decrease in FM in both groups. A continuous weight loss up to 8–12 months post-treatment in HNSCC survivors has previously been reported [Citation26,Citation27], and particularly Larsson et al. [Citation27] reported an increasing weight loss from 6.8% at the end of treatment to 17.4% one year post-treatment in 20 HNSCC survivors. Thus, one could speculate that the intervention may have had a positive effect by counteracting a potential continuous loss of BW.
Changes in muscle strength and functional performance
We report significant changes in isometric and isokinetic muscle strength in both groups following PRT with average increases of about 20%. Studies on PRT in other cancer survivors report strength changes ranging from 10% to 110% [Citation9]. These heterogeneous results are probably caused by different training modalities, duration of intervention, cancer types and methods of evaluation.
The observed improvements in functional performance are in accordance with a previous study on PRT in cancer survivors [Citation28]. Despite the lack of a control group, this indicates that the increased muscle mass and strength gains following PRT translates into an improved functional performance in both groups, which we consider clinically relevant and perhaps for the patients the most meaningful effect of PRT.
Strengths and limitations
The advantage of the minimally supervised approach to PRT is that this more realistically reflects how training in a local community setting may be implemented and thus, the feasibility recognized in our scientifically controlled set up may be more easily transferable to future radiotherapy treated HNSCC patients engaging in PRT. A limitation of the minimally supervised approach of the PRT is difficulties in controlling and ensuring identical relative training loads and progressions between patients and groups.
No significant additional effect of protein and creatine supplementation was observed although this finding should be interpreted cautiously due to the low number of participants in this study. Furthermore, we observed only a borderline significant increase over time in LBM in the PLA group. As discussed earlier, the relatively large dropout rate of 30% (especially from the PLA group) reduced the power of the statistical analyses, whereby these results may be due to type II errors.
Being a feasibility study we did not include a control group in our design, which is an obvious limitation of the study with respect to determining the efficacy of the interventions. One could argue that LBM, muscle strength and functional performance would increase through general improvements following the three months. Thus, to validate the positive findings from this study further controlled studies are needed.
Conclusion
PRT interventions can be performed in HNSCC patients safely and with a high adherence and completion rate. The results show overall increases over 12 weeks in LBM, strength and functional performance in the patients completing the training. Further controlled studies are required to determine whether these effects are caused by the training as such or are a consequence of spontaneous recovery.
Supplementary Appendix 1–5
Download PDF (204.4 KB)Acknowledgements
We acknowledge patient coordinators Berit Eide and Ditte Møller Nielsen for their invaluable assistance in patient recruitment and training organization.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Simon Lønbro was funded by the Danish Head and Neck Cancer Group (DAHANCA), the Lundbeck Center for Interventional Research in Radiation Oncology (CIRRO), the Danish Cancer Society and Aarhus University. The project was further supported by Beckett Fonden, Arvid Nillsons Fond, Dansk Kraeftforskningsfond and Andersen-Isted Fonden.
References
- Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck 2007;29:893–900.
- Langius JA, Kruizenga HM, Uitdehaag BM, Langendijk JA, Doornaert P, Leemans CR, . Resting energy expenditure in head and neck cancer patients before and during radiotherapy. Clin Nutr Epub 2012 Jan 24.
- Oates JE, Clark JR, Read J, Reeves N, Gao K, Jackson M, . Prospective evaluation of quality of life and nutrition before and after treatment for nasopharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2007;133:533–40.
- Brookes GB. Nutritional status – a prognostic indicator in head and neck cancer. Otolaryngol Head Neck Surg 1985;93:69–74.
- Datema FR, Ferrier MB, Baatenburg de Jong RJ. Impact of severe malnutrition on short-term mortality and overall survival in head and neck cancer. Oral Oncol 2011;47:910–4.
- Jager-Wittenaar H, Dijkstra PU, Vissink A, Langendijk JA, van der Laan BF, Pruim J, . Changes in nutritional status and dietary intake during and after head and neck cancer treatment. Head Neck 2011;33:863–70.
- Andersen AH, Vinther A, Poulsen LL, Mellemgaard A. Do patients with lung cancer benefit from physical exercise? Acta Oncol 2011;50:307–13.
- Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, . American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc 2010;42:1409–26.
- De BI, Schep G, Backx FJ, Vreugdenhil G, Kuipers H. Resistance training in cancer survivors: A systematic review. Int J Sports Med 2009;30:703–12.
- Dalton SO, Bidstrup PE, Johansen C. Rehabilitation of cancer patients: Needed, but how? Acta Oncol 2011;50:163–6.
- Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med 2004;34:329–48.
- Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol 2011;50:167–78.
- McNeely ML, Parliament MB, Seikaly H, Jha N, Magee DJ, Haykowsky MJ, . Effect of exercise on upper extremity pain and dysfunction in head and neck cancer survivors: A randomized controlled trial. Cancer 2008;113:214–22.
- Andersen LL, Tufekovic G, Zebis MK, Crameri RM, Verlaan G, Kjaer M, . The effect of resistance training combined with timed ingestion of protein on muscle fiber size and muscle strength. Metab Clin Experiment 2005;54:151–6.
- Esmarck B, Andersen JL, Olsen S, Richter EA, Mizuno M, Kjaer M. Timing of postexercise protein intake is important for muscle hypertrophy with resistance training in elderly humans. J Physiol 2001;535(Pt 1):301–11.
- Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, . Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr 2009;89:608–16.
- Rawson ES, Volek JS. Effects of creatine supplementation and resistance training on muscle strength and weightlifting performance. J Strength Cond Res 2003;17:822–31.
- Candow DG, Chilibeck PD. Timing of creatine or protein supplementation and resistance training in the elderly. Appl Physiol Nutr Metab 2008;33:184–90.
- Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: A systematic review. Cancer Treat Rev 2010; 36:185–94.
- McNeely ML, Parliament M, Courneya KS, Seikaly H, Jha N, Scrimger R, . A pilot study of a randomized controlled trial to evaluate the effects of progressive resistance exercise training on shoulder dysfunction caused by spinal accessory neurapraxia/neurectomy in head and neck cancer survivors. Head Neck 2004;26:518–30.
- Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev 2005;14:1672–80.
- Herrero F, San Juan AF, Fleck SJ, Balmer J, Perez M, Canete S, . Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. Int J Sports Med 2006;27:573–80.
- Eliot KA, Knehans AW, Bemben DA, Witten MS, Carter J, Bemben MG. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J Nutr Health Aging 2008; 12:208–12.
- Campbell WW, Leidy HJ. Dietary protein and resistance training effects on muscle and body composition in older persons. J Am Coll Nutr 2007;26:696S–703S.
- Lassen P. The role of human papillomavirus in head and neck cancer and the impact on radiotherapy outcome. Radiother Oncol 2010;95:371–80.
- van den Berg MG, Rasmussen-Conrad EL, van NL, van Binsbergen JJ, Merkx MA. A prospective study on malnutrition and quality of life in patients with head and neck cancer. Oral Oncol 2008;44:830–7.
- Larsson M, Hedelin B, Johansson I, Athlin E. Eating problems and weight loss for patients with head and neck cancer: A chart review from diagnosis until one year after treatment. Cancer Nurs 2005;28:425–35.
- Peddle-McIntyre CJ, Bell G, Fenton D, McCargar L, Courneya KS. Feasibility and preliminary efficacy of progressive resistance exercise training in lung cancer survivors. Lung Cancer 2012;75:126–32.