Abstract
Background. Given the longevity of thyroid cancer patients, any impairment in health-related quality of life (HRQoL) during the follow-up period is of considerable concern. Therefore, the first aim of this study was to assess (thyroid cancer specific) HRQoL among long-term thyroid cancer survivors and to compare this with the HRQoL of an age- and sex-matched normative population. Secondly, our aim was to investigate which clinical and socio-demographic characteristics and thyroid cancer specific problems were associated with HRQoL. Material and methods. All patients diagnosed with thyroid cancer between 1990 and 2008, as registered in the Eindhoven Cancer Registry, received a survey on HRQoL (EORTC QLQ-C30) and disease-specific symptoms (THYCA-QoL). The scores were compared with age- and sex-matched cancer free controls (n = 800). A series of multiple linear regression analyses were conducted to investigate the independent associations between clinical, socio-demographic and thyroid cancer specific factors with HRQoL. Results. A total of 306 patients (86%) responded to the invitation. Thyroid cancer survivors had significantly lower scores on physical, role, emotional, cognitive and social functioning (p < 0.001) compared to the normative population after adjusting for comorbidities. Sympathetic problems [feeling chilly (52%), hot flushes (40%)], neuromuscular problems [cramp legs (43%) and pain joints/muscles (64%)] and abrupt attacks of fatigue (50%) were the most often reported thyroid cancer specific complaints. Thyroid cancer specific neuromuscular, concentration, sympathetic and psychological problems explained 41–58% of the variance in HRQoL. Clinical and socio-demographic factors explained a small part of the variance in (thyroid cancer specific) HRQoL (1–27%). Conclusion. Long-term thyroid cancer survivors experience more symptoms and deteriorated HRQoL compared to the normative population. Thyroid cancer specific neuromuscular, sympathetic, concentration and psychological symptoms are stronger associated with HRQoL than clinical and socio-demographic factors alone. Awareness of these specific determinants of HRQoL could help health care practitioners to provide better supportive care.
In the Netherlands, the incidence of thyroid cancer (TC) is 3.1 cases per 100 000 people per year. Each year 570 patients are diagnosed with TC [Citation1]. Worldwide incidence rates are rising [Citation2]. The incidence is 2.5 times higher in women than in men. The papillary and follicular type account for about 80–90% of all cases, and poorly differentiated (medullary and anaplastic) TCs for about 10– 20%. As a result of the very good prognosis of papillary or follicular TC (exceeding > 90% five-year survival rates) the number of TC survivors is growing [Citation3].
Treatment of TC involves surgery, predominantly (near-)total thyroidectomy with or without lymphadenectomy, followed by radioiodine (iodine-131) therapy to ablate the remaining thyroid tissue. Depending on type and size of the tumor hemithyroidectomy could suffice. The removal of the whole thyroid gland is accompanied by a lifelong dependence on supplement therapy with thyroid hormone (levothyroxine), in the first years with dosing regimes suppressing thyroid stimulating hormone (TSH) production [Citation4]. Despite the efficacy of these primary treatments and the high long-term survival rates, 15–35% of the patients develop a locoregional recurrence or distant metastases, even decades later [Citation5]. Therefore, intensive long-term periodical follow-up is necessary.
Given the longevity of TC patients, possible impairments in health-related quality of life (HRQoL) during the follow-up period are of considerable concern. There are a limited number of studies focusing on HRQoL among TC survivors that moreover show contradictory results [Citation6]. A few studies found a lower HRQoL for TC survivors compared with a reference group, whereas other studies found similar HRQoL levels [Citation6]. The main problem of previous studies is the lack of a valid, specific instrument to assess the HRQoL among TC survivors. Therefore, we recently developed a TC specific HRQoL questionnaire to unveil specific TC related complaints [Citation7]. Furthermore, most other studies were inconclusive because they had small sample sizes and focused on short-term TC survivors (< 2 years after diagnosis) [Citation6]. Consequently, little is known about long-term HRQoL in TC patients. Therefore, the primary objective of our study was to assess general and TC specific HRQoL of long-term TC survivors and to compare this with an age- and sex-matched normative population. To be able to tailor supportive care to the individual needs of TC patients, it is important to obtain insight into the factors influencing HRQoL. Therefore our second aim was to investigate which clinical and socio-demographic characteristics and TC specific problems were associated with HRQoL.
Methods
Setting and population
This study is a population-based survey among TC survivors registered within the Eindhoven Cancer Registry (ECR) of the Comprehensive Cancer Centre South (CCCS). The ECR compiles data of all individuals newly diagnosed with cancer in the southern part of the Netherlands, an area with 10 hospitals serving 2.3 million inhabitants [Citation8]. All individuals diagnosed with TC between 1990 and 2008 as registered in the ECR were eligible for participation (n = 568) (). We excluded patients who had cognitive impairment or were too ill at time of the study (n = 31), had unverifiable addresses (n = 90), or died prior to the start of the study (according to the ECR, hospital records, and the Central Bureau for Genealogy) (n = 6). One hospital declined to participate (n = 86). The study was approved by the Medical Ethics Committee of the Maxima Medical Centre Eindhoven.
Data collection
Data collection was conducted in November 2010 within PROFILES (Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship). PROFILES is a registry for the study of the physical and psychosocial impact of cancer and its treatment from a dynamic, growing population-based cohort of both short- and long-term cancer survivors. PROFILES contains a large web-based component and is linked directly to clinical data from the ECR. Survivors were informed about the survey via a letter from their (ex)-attending specialist. The letter explained that by completing the questionnaire (online or by pencil-and-paper), patients consented to participate and agreed to the linkage of the questionnaire data with their disease history in the ECR. Further details of the data collection method have been described previously [Citation9]. Data from the PROFILES registry will be available for non-commercial scientific research, subject to study question, privacy and confidentiality restrictions, and registration (www.profilesregistry.nl).
Study measures
Socio-demographic and clinical characteristics.
Clinical information of the survivors was available from the ECR. The ECR routinely collects data on tumor characteristics, including the date of diagnosis, disease stage, primary treatment, and patient background characteristics including date of birth.
Questions on marital status, educational level, and current occupation were added to the questionnaire. Comorbidity at the time of survey was categorized according to an adapted Self-administered Comorbidity Questionnaire (SCQ) [Citation10], including heart disease, stroke, high blood pressure, long disease, diabetes, ulcer or stomach disease, kidney disease, anemia or other blood disease, depression, osteoarthritis, back pain, rheumatoid arthritis and a question on other medical problems.
EORTC-QLQ-C30.
HRQoL was measured by the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-C30) [Citation11]. This 30-item HRQoL questionnaire consists of five functional scales (physical, role, cognitive, emotional and social), a global quality of life scale, three symptom scales (fatigue, pain, nausea and vomiting) and a number of single items assessing common symptoms (dyspnea, loss of appetite, sleep disturbance, constipation and diarrhea) and perceived financial impact of the disease. The time frame of the questions is the previous week, and each item is scored on a four-point response scale ranging from 1, ‘not at all’ to 4, ‘very much,’ with the exception of the global QoL scale, which is scored on a seven-point modified linear analogue scale ranging from 1, ‘very poor’ to 7, ‘excellent’. After linear transformation, all scales and single item measures range in score from 0–100. A higher score on the functional scales and global QoL means better functioning and HRQoL, whereas a higher score on the symptom scales means more complaints.
Thyroid cancer specific HRQoL (THYCA-QoL).
TC specific HRQoL was measured by a self-developed questionnaire (THYCA-QoL) to assess side effects due to TC or its treatment [Citation7]. We developed and validated the TC specific HRQoL questionnaire according to the methods of the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life group. The questionnaire consists of 24 items, with a time frame of the previous week (except for the sexual interest item which is four weeks) and each item is scored on a four-point response scale ranging from 1, ‘not at all’ to 4, ‘very much’. The THYCA-QoL consists of seven symptom scales (neuromuscular, voice, concentration, sympathetic, throat/mouth, psychological and sensory problems) and six single items (problems with scar, felt chilly, tingling hands/feet, gained weight, headaches, interest in sex). Scores were linear transformed to a 0–100 scale. A higher score on this scale means more symptoms.
Normative population
Normative population data was obtained from CentERpanel, an online household panel that is representative of the Dutch population. The process of the annual data collection, which started in 2009 by our study group, is described elsewhere [Citation12]. The data wave in 2009 included assessment of HRQoL (EORTC QLQ-C30). From the 1731 members ≥ 18 years who responded (78%), an age- and sex-matched normative cancer-free sample (n = 800) was selected for this study to reflect the age and sex distribution of the TC sample. Socio-demographic data were also collected for this group.
Statistical analyses
Differences in clinical and socio-demographic characteristics between respondents, non-respondents and patients with unverifiable addresses and between TC patients and the normative population were compared using χ2 statistics for categorical variables and t-tests or analysis of variance (ANOVA) for continuous variables.
ANCOVA was used to compare the mean scores on the individual EORTC QLQ-C30 scales between TC survivors and the normative population, controlled for the number of comorbidities. In addition, clinical meaningfulness of the differences was determined according to the guidelines of the EORTC Quality of Life Group [Citation13]. Differences in (TC specific) HRQoL between subgroups of survivors (< 5 years; 5–10 years; > 10 years since diagnosis) were analyzed using ANOVA.
A series of multiple linear regression analyses (enter method) were conducted to investigate the independent associations between clinical (disease stage, primary treatment, comorbidity) and socio-demographic characteristics (age, sex, partner, educational level) with subscale scores of the EORTC-QLQ-C30 and THYCA-QoL. Also, independent associations of the TC specific problems with the subscales of the EORTC QLQ-C30 were analyzed with multiple linear regression analyses and were adjusted for clinical and socio-demographic variables (determined a priori).
All statistical analyses were performed using SPSS version 17.0 (Statistical Package for Social Sciences, Chicago, IL, USA) and p-values < 0.05 were considered statistically significant.
Results
Patient and tumor characteristics
Three hundred and six patients returned a completed questionnaire (response 86%). A comparison between respondents, non-respondents and patients with unverifiable addresses indicated that patients with unverifiable addresses were younger compared to non- respondents and respondents. No differences between groups were seen regarding sex, type of TC, stage of the disease or primary treatment. Socio-demographic and clinical characteristics are described in .
Table I. Socio-demographic and clinical characteristics of questionnaire respondents and normative population.
HRQoL of TC survivors compared with normative population
HRQoL of TC survivors as measured by the EORTC-QLQ-C30 was compared with an age- and sex-matched normative sample from the general Dutch population (a and b). The survivors had significantly lower scores on physical, role, emotional, cognitive and social functioning compared to the normative population after adjusting for comorbidities. No differences were seen in global health. The differences between TC survivors and normative population were of small clinical importance for the subscales physical, role and social functioning and medium for cognitive functioning and could not be determined for the emotional functioning scale [Citation13].
Figure 2. HRQoL of thyroid cancer survivors compared to normative population. (a) Mean EORTC-QLQ-C30 functioning subscale scores (0–100) of survivors in comparison with a Dutch normative population. A higher score means better functioning. An asterisk above the bar indicates a group difference (ANCOVA, p < 0.05, adjusted for comorbidity). (b) Mean EORTC-QLQ-C30 symptom subscale scores (0–100) of survivors in comparison with a Dutch normative population. A higher score indicates more symptoms. An asterisk above the bars indicates a group difference (ANCOVA, p < 0.05, adjusted for comorbidity).
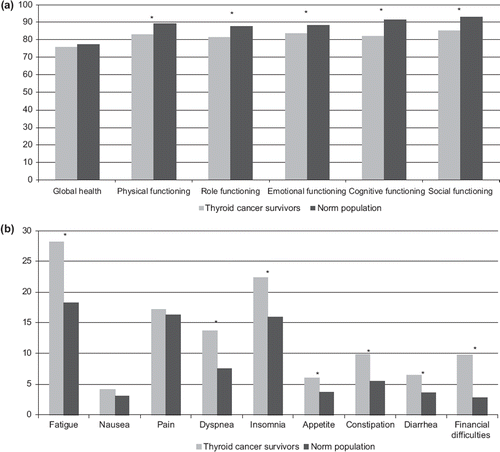
TC survivors had significantly more symptoms of fatigue, dyspnea, insomnia, appetite, constipation, diarrhea and had higher scores on financial difficulties compared to the normative population after adjusting for comorbidities. No differences were seen for nausea and pain. The differences were of small clinical importance for fatigue, dyspnea, insomnia and financial difficulties and of no clinical importance for appetite, constipation and diarrhea.
Thyroid cancer specific HRQoL
The majority of TC survivors experienced disease specific symptoms during the past week. Sympathetic problems [feeling chilly (52%), hot flushes (40%)], neuromuscular problems [cramp legs (43%) and pain joints/muscles (64%)] and abrupt attacks of fatigue (50%) were the most often reported complaints.
Clinical and socio-demographic factors associated with (thyroid cancer specific) HRQoL
When stratified by years since diagnosis (< 5 years; 5–10 years; > 10 years since diagnosis) no differences between the groups were seen in functioning and symptom scales of the EORTC QLQ-C30 (). TC survivors > 10 years since diagnosis reported less TC specific voice, concentration, sympathetic, throat/mouth problems compared to survivors < 5 years since diagnosis (p < 0.05). Survivors < 5 years since diagnosis reported more problems with their scar compared to survivors 5–10 years and > 10 years since diagnosis (p < 0.05).
Table II. Mean scores (±SD) of thyroid cancer survivors on EORTC QLQ-C30 and THYCA-QoL scales (0–100) and single items according to years since diagnosis.
Multiple linear regression analyses showed that clinical factors were not associated with the functional and symptom scales of the EORTC QLQ-C30, except for disease stage which was negatively associated with emotional functioning (). Having one or more comorbidities was associated with worse global health and physical, role and emotional functioning and more fatigue (Beta = 0.24; p < 0.05), pain (Beta = 0.25; p < 0.05), dyspnea (Beta = 0.18; p < 0.05), insomnia (Beta = 0.17; p < 0.05) and less appetite (Beta = 0.15; p < 0.05). Higher age was associated with worse physical functioning and better emotional functioning. Absence of a partner and lower educational level were associated with worse functioning and more symptoms (Betas ranging from 0.12–0.24; p < 0.05).
Table III. Standardized betas of multiple linear regression analyses evaluating the association of independent variables with the HRQoL scales.
Most clinical variables were not associated with TC specific problems (), however fewer years since diagnosis was associated with more voice, throat and scar problems (Beta = −0.14; p < 0.05). Medullar TC and a higher stage disease were associated with more sympathetic problems. More comorbidities, absence of a partner and lower educational level were associated with more TC specific symptoms. The explained variance (R2) of clinical and socio-demographic variables ranged from 1% to 27%.
Table IV. Standardized betas of multiple linear regression analyses evaluating the association of independent variables with the thyroid cancer specific symptom scales.
Multiple linear regression analyses, corrected for socio-demographic and clinical characteristics showed that more neuromuscular problems were associated with worse global health (Beta = −0.23; p < 0.05), physical (Beta = −0.35; p < 0.01), role (Beta = −0.31; p < 0.01), emotional (Beta = −0.16; p < 0.05) and social functioning (Beta = −0.36; p < 0.01). More concentration problems were associated with worse cognitive (Beta = −0.73; p < 0.01) and social functioning (Beta = −0.23; p < 0.01), while more sympathetic problems were associated worse global health (Beta = −0.17; p < 0.05). More psychological problems were associated with worse physical (Beta = −0.25; p < 0.05), emotional (Beta = −0.53; p < 0.01) and social functioning (Beta = −0.22; p < 0.01) and worse global health (Beta = −0.19; p < 0.05). The explained variance (R2) ranged from 41% for global health and 58% for cognitive functioning.
Discussion
Our findings showed that TC survivors report statistical significant and clinical relevant lower levels of physical and psychosocial functioning, and significantly more symptoms compared to an age- and sex-matched normative population. TC specific neuromuscular, concentration, sympathetic and psychological problems were most strongly associated with worse physical and psychosocial functioning.
Primary treatment was not associated with HRQoL, which is in agreement with an earlier study that found no differences in HRQoL scores between patients who underwent total thyroidectomy and hemithyroidectomy, respectively [Citation14]. A recent study showed that those patients submitted to higher doses of radioactive iodine are at risk for poor HRQoL [Citation15]. For part of the patients there may be overtreatment, since low-risk patients are unlikely to benefit from radioactive iodine therapy after initial surgery [Citation16]. Unfortunately, no data on doses of radioactive iodine were available in the present study. Furthermore, the recent use of recombinant human thyroid stimulating hormone (rhTSH) as alternative for thyroid hormone withdrawal preserved HRQoL during radioactive iodine ablation [Citation6].
Disease stage and time since diagnosis were not related to HRQoL, although high disease stage and short time since diagnosis were related to more TC specific voice and throat/mouth problems, indicating the severity of surgery. Our results show that TC specific problems related to thyroid dysregulation (neuromuscular, concentration, sympathetic, psychological and sensory problems) were most strongly associated with HRQoL. Although TC specific symptoms diminish over time, they remain present long after initial treatment. The high doses of replacement therapy (TSH < 0.1 mU/L) in the first years of follow-up, for patients who underwent ablation therapy or had high risk of recurrence, and the subsequent TSH suppression may cause symptoms of subclinical hyperthyroidism. After the first years the doses of replacement therapy will be lowered for low-risk survivors [TSH level around median (1 mU/L)], however, the lower doses might not be the optimal (pre-operative) level for the specific patient and in that case the symptoms are a result of the supplemental thyroid hormone treatment [Citation17]. Furthermore, long-term exposure to excess thyroid hormone can lead to (irreversible) changes of the autonomous nervous system, which could not be reversed by restoration to euthyroidism [Citation18]. These changes possibly influence the well-being of a patient and might be an explanation for the symptoms patients experience even long-term after diagnosis.
Having one or more comorbidities was associated with worse functioning and more (TC specific) symptoms. Comorbid conditions could affect the presentation and recognition of symptoms and result in worse HRQoL on their own. Since 78% of the survivors reported one or more comorbidities, it is unclear whether comorbidity is associated with lower HRQoL or whether it is the disease itself, or their collaborative effect that negatively impacts HRQoL. Since the prevalence of comorbidities in the normative population (natural aging effects) was significantly lower compared to TC survivors, this might indicate that part of the comorbid diseases developed after primary treatment for TC and their development might be related to the treatment procedures [Citation15]. Studies suggest that subclinical hyperthyroidism (due to suppressive hormone therapy) has potential harmful effects on bone metabolism and the cardiovascular system [Citation19] and is even associated with increased mortality due to cardiovascular diseases [Citation20]. More research is needed into the type, severity and impact of comorbid conditions in TC survivors, the development over time and the associations with treatments [Citation21].
Our finding that partnered patients and patients with a higher educational level had higher HRQoL scores are in line with previous research [Citation22,Citation23]. Partnered patients experience more social support [Citation23] and higher educated patients possibly receive more information by asking more questions, are more satisfied, choose the best doctor for their feeling and have a better understanding of the disease [Citation22], that might lead to lower levels of anxiety and depression and better coping strategies.
The strengths of our population-based study are the high response rate, the inclusion of long-term TC survivors (2–20 years after diagnosis), the large sample size, the availability of normative data of the EORTC QLQ-C30 and the use of a TC specific HRQoL questionnaire. However, the present study has the following limitations. First, detailed follow-up treatment data was lacking because the cancer registry registers only the primary treatment procedure. Secondly, patients with unverifiable addresses were younger and more often had stage I papillary tumor compared to the respondents. Therefore our findings cannot be generalized to this younger group of patients with papillary tumors. Furthermore, people from the normative population more often had a higher educational level compared to the TC survivors, which could have influenced our results. The fourth limitation was the cross-sectional study design which limits the determination of causal associations. Assessment of more specific biochemical (TSH levels and serum free T4, T3) and treatment parameters (levothyroxine dose, extent surgery, additional treatments) in combination with the assessment of THYCA-QoL over time is needed to draw definitive conclusions about the exact mechanisms leading to a decreased HRQoL among long-term TC survivors.
As a consequence of the good prognosis for differentiated TCs, patients are sometimes advised by HCP that TC is not a serious problem [Citation24]. This attitude trivializes the importance of the disease and causes patients to feel dismissed as not having a serious disease [Citation25]. Since survivors report decreased HRQoL and several TC specific symptoms long-term after diagnosis, there is a need for multidisciplinary aftercare. The first step for multidisciplinary aftercare will be recognition, recording and acknowledgement of specific long-term health problems of patients with TC by HCPs. Our questionnaire (THYCA-QoL) can be of value in the treatment and after care of TC survivors. It will make HCPs more aware of the potential HRQoL issues TC survivors are dealing with. HCPs can monitor changes in HRQoL (and its determinants) over time and provide continuous support for patients with TC during follow-up surveillance. In addition, HCPs could also help to improve HRQoL among patients with TC by showing them how to manage uncomfortable symptoms and by referring them for further services when necessary (e.g. physical activity programs or support groups).
In conclusion, TC survivors have a worse HRQoL compared to the normative population. TC specific neuromuscular, sympathetic, concentration and psychological problems last long after diagnosis and are more strongly associated with HRQoL than socio-demographic and clinical factors alone. Awareness of these specific determinants of HRQoL could help healthcare practitioners to provide better supportive care.
Acknowledgements
We would like to thank all patients and their doctors from the following hospitals for their (ongoing) participation in this study: Bernhoven Hospital, Veghel and Oss; Catharina Hospital, Eindhoven; Elkerliek Hospital, Helmond and Deurne; Jeroen Bosch Hospital, Den Bosch; Maxima Medical Centre, Eindhoven and Veldhoven; Sint Anna Hospital, Geldrop; St. Elisabeth Hospital, Tilburg; Twee Steden Hospital, Tilburg; VieCuri Hospital, Venlo and Venray. The data collection of this study was funded by the Comprehensive Cancer Centre South, Eindhoven, The Netherlands, and a Medium Investment Grant from the Netherlands Organisation for Scientific Research (NWO#480-08-009). Dr. Floortje Mols is supported by a VENI grant (#451-10-041) from the Netherlands Organization for Scientific Research (The Hague, The Netherlands), Dr. Lonneke van de Poll-Franse is supported by a Cancer Research Award from the Dutch Cancer Society (#UVT-2009–4349). The contribution of LM Buffart was supported by a fellowship granted by the EMGO Institute for Health and Care Research. These funding agencies had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Dutch cancer figures. [cited 2012 Sep 24]. Available from: http://www.cijfersoverkanker.nl
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002.JAMA 2006;295:2164–7.
- Davies L, Welch HG. Thyroid cancer survival in the United States: Observational data from 1973 to 2005.Arch Otolaryngol Head Neck Surg 2010;136:440–4.
- Pacini F, Castagna MG, Brilli L, Pentheroudakis G. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.Ann Oncol 2010;21(Suppl 5): v214–9.
- Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer.Am J Med 1994;97:418–28.
- Husson O, Haak HR, Oranje WA, Mols F, Reemst PH, van de Poll-Franse LV. Health-related quality of life among thyroid cancer survivors: A systematic review.Clin Endocrinol (Oxf) 2011;75:544–54.
- Husson O, Haak HR, Mols F, Nieuwenhuijzen GAP, Nieuwlaat WA, Reemst PH, . Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors.Acta Oncol Epub2012 Sep 27.
- Janssen-Heijnen MLG, Louwman WJ, Van de Poll-Franse LV, Coebergh JWW. Results of 50 years cancer registry in the South of the Netherlands: 1955–2004 (in Dutch). Eindhoven: Eindhoven Cancer Registry; 2005.
- van de Poll-Franse LV, Horevoorts N, Eenbergen MV, Denollet J, Roukema JA, Aaronson NK, . The Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship registry: Scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts.Eur J Cancer 2011;47:2188–94.
- Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: A new method to assess comorbidity for clinical and health services research.Arthritis Rheum 2003;49:156–63.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology.J Natl Cancer Inst 1993;85:365–76.
- van de Poll-Franse LV, Mols F, Gundy CM, Creutzberg CL, Nout RA, Verdonck-de Leeuw IM, . Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population.Eur J Cancer 2011;47:667–75.
- Cocks K, King MT, Velikova G, Martyn St-James M, Fayers PM, Brown JM. Evidence-based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30.J Clin Oncol 2011; 29:89–96.
- Shah MD, Witterick IJ, Eski SJ, Pinto R, Freeman JL. Quality of life in patients undergoing thyroid surgery.J Otolaryngol 2006;35:209–15.
- Almeida JP, Vartanian JG, Kowalski LP. Clinical predictors of quality of life in patients with initial differentiated thyroid cancers.Arch Otolaryngol Head Neck Surg 2009;135: 342–6.
- Brown RL, de Souza JA, Cohen EE. Thyroid cancer: Burden of illness and management of disease.J Cancer 2011;2: 193–9.
- Andersen S, Pedersen KM, Bruun NH, Laurberg P. Narrow individual variations in serum T(4) and T(3) in normal subjects: A clue to the understanding of subclinical thyroid disease.J Clin Endocrinol Metab 2002;87:1068–72.
- Eustatia-Rutten CF, Corssmit EP, Heemstra KA, Smit JW, Schoemaker RC, Romijn JA, . Autonomic nervous system function in chronic exogenous subclinical thyrotoxicosis and the effect of restoring euthyroidism.J Clin Endocrinol Metab 2008;93:2835–41.
- Utiger RD. Subclinical hyperthyroidism – just a low serum thyrotropin concentration, or something more?N Engl J Med 1994;331:1302–3.
- Parle JV, Maisonneuve P, Sheppard MC, Boyle P, Franklyn JA. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: A 10-year cohort study.Lancet 2001;358:861–5.
- Kuijpens JL, Janssen-Heijnen ML, Lemmens VE, Haak HR, Heijckmann AC, Coebergh JW. Comorbidity in newly diagnosed thyroid cancer patients: A population-based study on prevalence and the impact on treatment and survival.Clin Endocrinol (Oxf) 2006;64:450–5.
- Hirsch D, Ginat M, Levy S, Benbassat C, Weinstein R, Tsvetov G, . Illness perception in patients with differentiated epithelial cell thyroid cancer.Thyroid 2009;19: 459–65.
- Schultz PN, Stava C, Vassilopoulou-Sellin R. Health profiles and quality of life of 518 survivors of thyroid cancer.Head Neck 2003;25:349–56.
- Sipos JA, Mazzaferri EL. Thyroid cancer epidemiology and prognostic variables.Clin Oncol (R Coll Radiol) 2010;22: 395–404.
- Sawka AM, Goldstein DP, Brierley JD, Tsang RW, Rotstein L, Ezzat S, . The impact of thyroid cancer and post-surgical radioactive iodine treatment on the lives of thyroid cancer survivors: A qualitative study.PLoS One 2009;4: e4191.