Abstract
Introduction. Fatigue after treatment for breast cancer (BC) is common, but poorly understood. We examined the fatigue levels during first year after radiotherapy (RT) according to the extent of RT (local or locoregional), hormonal therapy (HT) and chemotherapy (CT). The impact of comorbidity was also explored. Moreover, we compared fatigue levels in patients with the general population (GenPop) data. Material and methods. BC patients (n = 250) referred for post-operative RT at St. Olavs Hospital, Trondheim, Norway, were enrolled. Fatigue was measured by the EORTC QLQ-C30-fatigue subscale, ranging from 0 to 100, before RT (baseline), after RT, and at three, six, and 12 months. Clinical and treatment-related factors were recorded at baseline. GenPop data was available from a previous survey (n = 652). Linear mixed models and analysis of covariance were applied. Results. Compliance ranged from 87% to 98%. At baseline, mean value (SD) of fatigue in BC patients was 26.8 (23.4). The level increased during RT (mean change 8.3, 95% CI 5.5–11.1), but declined thereafter and did not differ significantly from pre-treatment levels at subsequent time points. In age-adjusted analyses, locoregional RT accounted for more overall fatigue than local RT (mean difference 6.6, 95% CI 1.2–12.0), but the association was weakened and not statistical significant when adjusting for CT and HT. Similar pattern was seen for CT and HT. The course of fatigue differed significantly by CT (p < 0.001, interaction test). At baseline, fatigue levels were higher in patients with than without CT, but at subsequent time points similar levels were evident, indicating a temporary adverse effect of CT. Comorbidity was significantly associated with increased level of fatigue, independent of other factors (mean difference 8.1, 95% CI 2.2–14.1). BC-patients were not significantly more fatigued than GenPop, except for immediately after ending RT, and then only among those without comorbidity (mean 35.9 vs. 25.8, p < 0.001). Conclusion. Comorbidity seems to be a more important determinant for fatigue levels than the cancer treatment.
Advances in breast cancer (BC) treatment have led to improved survival and health-related quality of life (HRQoL) for BC patients [Citation1]. However, fatigue is still a common symptom in these patients [Citation2,Citation3]. Fatigue's association with impaired HRQoL [Citation4] and also with recurrence and reduced survival [Citation5] underlines the necessity of better understanding this symptom.
Fatigue in BC patients has mainly been studied in those treated with chemotherapy (CT) [Citation6], and less in patients treated with modern radiotherapy (RT), either alone or in addition to other adjuvant treatments [Citation2,Citation4,Citation7]. The course of fatigue during RT has been found to differ between patients pre-treated with CT (CT-RT-group) and those with no prior treatment (RT-group). Higher levels of fatigue at the start of RT, and a possible response shift in the CT-RT group, have been argued to influence this course [Citation7–9].
Contrasting results have been reported on post-treatment levels of fatigue. Several longitudinal studies have reported no significant association between type of adjuvant treatment and fatigue [Citation7,Citation10–12]. Others have reported that CT-RT patients are more fatigued than RT patients after the completion of RT and at six months after the end of treatment, indirectly assessed by comparing each patient group with healthy controls [Citation13]. Another study observed a significant increase in fatigue from six to 42 months after end of treatment among CT-RT patients, in contrast to RT patients, whose levels remained stable or decreased, and were significantly lower than the CT-RT group [Citation14].
Fatigue is strongly related to chronic non- malignant diseases such as heart and lung diseases, diabetes, and depression [Citation15,Citation16], which are prevalent both in BC patients [Citation3,Citation17,Citation18] and in general populations (GenPop) [Citation15,Citation16,Citation19]. Thus, fatigue in BC patients could be stronger associated to comorbid chronic conditions, rather than to the cancer and its treatment. Most studies [Citation3,Citation18,Citation20], though not all [Citation21], support the opinion that fatigue is more severe in BC patients than in GenPop subjects. Some investigators attempt to rule out other causes of fatigue by using strict inclusion or exclusion criteria [Citation12–14] or by making adjustments in statistical analyses [Citation18,Citation20]. However, specific focus on fatigue in comorbidity groups has been less frequently addressed [Citation16].
The diversity of designs, recruitment procedures, measurement tools and fatigue endpoints makes confident comparisons between studies challenging, and may explain the lack of consistent findings in reported levels and trajectories of fatigue (Appendix, available online, Overview of selected studies investigating fatigue during and/or after RT in BC patients). In addition, small sample sizes in several studies [Citation8,Citation11,Citation22,Citation23] lead to imprecise estimates. Finally, potential treatment effects assessed when former adjuvant treatment-regimes were applied [Citation10,Citation17, Citation18,Citation20,Citation21], may not be comparable to results from studies from more recent periods.
The present study aimed to explore whether modern RT, alone and in combination with adjuvant treatments, influenced the level and the course of fatigue in BC patients during RT and in the subsequent year. We also investigated whether comorbidity influenced this pattern. In addition, fatigue levels in BC patients were compared with data from the general Norwegian population.
Material and methods
Breast cancer patients
The present study is part of a large, prospective longitudinal study investigating HRQoL and adverse effects after RT in BC patients at St. Olavs University Hospital in Trondheim, Norway. Patients were consecutively included after offering informed consent if they: 1) were referred for post-operative local or locoregional RT – alone or in addition to CT; 2) had no metastatic disease; 3) had no physical or psychological disorders that would interfere with participation; and 4) were able to speak and understand Norwegian. Oral and written study information was provided at the first consultation prior to RT. Patients who developed metastatic diseases during follow-up were excluded. The study was approved by The Regional Committee for Medical Research Ethics and The Norwegian Data Inspectorate. The recruitment period lasted from February 2007 to October 2008. Of 261 eligible patients, 250 (96%) agreed to participate. The recruitment procedure is published elsewhere [Citation9].
Treatment
Standardized treatment was provided in accordance with national guidelines [Citation24]. CT was administered routinely prior to RT as six antracycline-based courses, or four antracycline-based courses followed by four courses of docetaxel. RT was planned by a three-dimensional (3D) computer tomography dose planning system (Oncentra Masterplan). In local RT, 50 Gy was delivered to the breast/chest wall in 2 Gy/fraction, five days a week. Locoregional RT also included 46 Gy delivered to lymph nodes in the periclavicular region ± the axillae. Patients < 40 years with lumpectomies received an additional boost dose of up to 66 Gy to the tumor bed.
Follow-up assessments
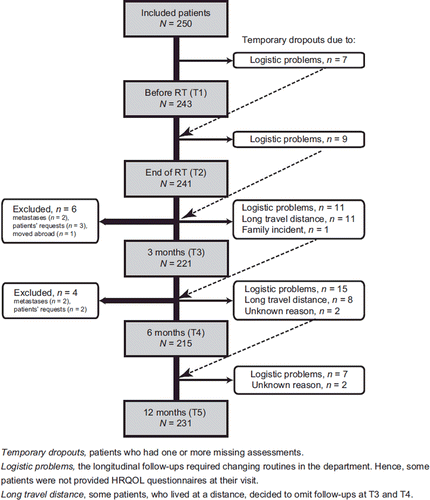
All assessments were conducted through extended out-patient follow-up at the hospital; before starting RT (T1), at completion of RT (T2) and three, six and 12 months after RT (T3–T5). At inclusion, some patients decided to omit T3 and T4 assessments due to long travel distances ().
At baseline (T1), treatment and clinical variables including comorbidity (cardiovascular diseases, pulmonary disorders, diabetes, or depression) were registered by the oncologist, and sociodemographic data was collected by self-report questionnaires. The employment status was registered at each assessment. Fatigue was measured by the three-item fatigue subscale of the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 [Citation25], addressing the symptoms of tiredness, weakness, and lack of energy during the previous week. Response options ranged from 1 (not at all) to 4 (very much). The fatigue scale was calculated according to the EORTC scoring manual as the average score and transformed to a 0–100 scale, with higher scores indicating more fatigue. The scale is recommended for use in larger HRQoL trials [Citation26], and has been independently validated as a separate measure in Norwegian cancer survivors [Citation27]. Internal reliability [Citation19] and stability over time [Citation16] in the Norwegian GenPop has been documented. Cronbanch’s alpha in the present samples ranged from 0.90-0.87 (T1-T5) for BC patients and was 0.80 for the female GenPop. A mean score difference of 5–10 is usually regarded as a small but clinically noticeable change, a change between 10 and 20 as moderate, and > 20 as a large clinical change [Citation28].
General population
Data from the Norwegian GenPop on the EORTC QLQ-C30 was obtained from a survey conducted in 1996 [Citation19]. Original data from the female population (n = 949, response rate 69%) was available to the authors. Self-reported information on chronic diseases, including the presence or history of cardiovascular diseases, pulmonary disorders, or diabetes, was used as a measure of comorbidity. Respondents younger than 29 years (one patient, 184 GenPop) and older than 79 years (two patients and 49 GenPop) were excluded in order to achieve similar age distributions when comparing the samples. GenPop subjects with a cancer diagnosis were also excluded (n = 72), yielding 652 subjects eligible for analyses.
Statistical analyses
Changes in mean levels of fatigue over time in patients were examined by means of a Linear Mixed Model (LMM). LMM provides great flexibility and allows for missing values at single time points; all available data is thus utilized [Citation29]. Patients were defined as random factor, whereas potential predictors for fatigue levels were defined as fixed factors (random intercept model). An autoregressive covariance structure (AR1) was defined to take account of dependency in the repeated measurements of fatigue. The time of assessment was modeled as a categorical variable to allow for a non-linear time pattern. Post-treatment levels of fatigue (T2–T5) were compared with pre-treatment level (T1), and each person thus served as its own control. RT (locoregional, local), CT (yes, no), hormonal treatment (HT) (yes, no) and comorbidity (yes, no) were also included as categorical factors. The Restricted Maximum Likelihood (REML) procedure was applied for estimation of regression coefficients. To examine whether changes in fatigue over time differed according to treatment or comorbidity, or whether the overall association with the extent of RT differed between patients with and without CT, HT as well as comorbidity, interaction terms were included in the model one at a time. In case of (borderline) significant interactions, results specific to subgroups were reported. All analyses were adjusted for age, included in the model as a continuous variable (linear trend through one year interval). Analyses of covariance (ANCOVA) were applied to compare mean levels of fatigue among BC patients at each time point with mean levels of fatigue in the Norwegian GenPop. Adjustments were made for age and comorbidity. Separate analyses were conducted for subjects with and without comorbidity. All statistical analyses were carried out using IBM SPSS Statistics 19.0 for Windows.
Results
Compliance and patient characteristics
Compliance rates at each assessment were 98% (T1), 97% (T2), 90% (T3), 87% (T4), and 94% (T5). Two hundred and fifty patients were included, and during follow-up 62 patients had missing information at one or more assessments (temporary dropouts) and 10 patients were excluded at T3 and T4 (). Sociodemographic and clinical patient characteristics are provided in and .
Table I. Clinical characteristics of patients (N = 250).
Table II. Demographic characteristics of patients and the general population (GenPop).
Time pattern and differences in mean fatigue scores by adjuvant treatments, and comorbidity
At baseline, mean value (SD) of fatigue in BC patients was 26.8 (23.4). Observed mean levels of fatigue (95% CI) during follow-up (T1–T5), in subgroups of adjuvant treatments and comorbidity, are shown in . The level of fatigue changed significantly over time (p < 0.001, ), but the time-specific estimates indicated a temporary increase of clinical importance only during RT (mean difference 8.3). At three, six, and 12 months after RT, the overall level of fatigue was not significantly different from the baseline level.
Figure 2. Observed mean score of fatigue with 95% CI during the first year after radiotherapy (T1–T5) in groups defined by adjuvant treatments and comorbidity.
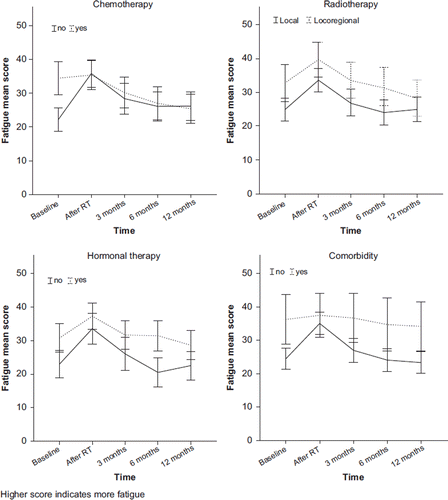
Table III. Differences in mean scores of fatigue by time, adjuvant treatments and comorbidity.
In age-adjusted analyses, locoregional RT accounted for more overall fatigue than local RT (, Model 1, mean difference 6.6, p = 0.017), but in analyses adjusted for CT and HT, no significant associations were found (Model 2). Similar pattern was seen for CT and HT. Comorbidity was significantly associated with higher level of fatigue, almost independent of treatment factors (, Model 3, mean difference 8.1, adjusted model, p = 0.008).
Interaction effects
The overall association between the extent of RT (locoregional, local) and fatigue levels did not differ significantly by treatment of CT or by presence of comorbidity (p = 0.70 and p = 0.66), but the interaction with HT was borderline significant (p = 0.08). In patients without HT, locoregional RT seem to account for more fatigue than local RT (mean difference 12.2, 95% CI 0.9–23.5), whereas no differences were present in patients receiving HT (mean difference 0.97, 95% CI -5.8–7.7). The course of fatigue did not differ significantly according to extent of RT, HT, or comorbidity (p = 0.56, 0.13 and 0.11, respectively, interaction tests), but differed between patients with and without CT (p < 0.001). Separate analyses were therefore performed for patients with and without CT. We also performed analyses in subgroups defined by comorbidity, since the observed mean values appeared to follow a different pattern (), though we did not have sufficient power to detect an interaction with time. Only results for RT, and change over time, are shown.
Results from subgroup analyses ()
Patients with no prior CT had a marked increase in fatigue during RT (B = 13.8, p < 0.001), followed by a slow decrease, though levels remained significantly higher than baseline at all time points. The mean score of fatigue did not differ significantly by the extent of RT. Patients pre-treated with CT had significantly higher levels of fatigue at T1 than those without CT (mean 34.4 vs. 22.0, p < 0.001, two sample t-test), and the time-specific estimates within this subgroup showed no further increase during RT. After T2, fatigue decreased steadily and was significantly lower than the baseline level at six and 12 months after RT. In the period from T2 to T5, fatigue levels were rather similar in patients with and without CT (). In the CT-RT group, locoregional RT (n = 56) accounted for more overall fatigue than local RT (n = 47), but not significantly (B = 5.1, p = 0.17).
Table IV. Differences in mean scores of fatigue by time and extent of radiotherapy in subgroups defined by chemotherapy and comorbidity.
Patients without comorbidity had a significant change in fatigue during follow-up (p < 0.001), shown by a clinically significant increase during RT (mean difference 10.3) followed by a prompt decline back to the baseline level at three months after RT, remaining stable at six and 12 months. In this group, locoregional RT (n = 61) accounted for more overall fatigue than local RT (n = 129), but not significantly (B = 5.4, p = 0.11). Patients with comorbidity had a significantly higher level of baseline fatigue than patients without a comorbid condition (mean 36.3 vs. 24.5, p = 0.005, two sample t-test), and had no overall change in fatigue during follow-up (p = 0.92). The extent of RT had no significant impact on the levels of fatigue among patients with comorbidity.
Breast cancer patients compared with the general population
Patients were significantly older (57.8 vs. 52.2, p < 0.001), less likely to have a comorbidity (23% vs. 32%, p = 0.001), and more likely to be married (77% vs. 63%, p < 0.001) than GenPop subjects ().
At the end of RT (T2), BC patients were significantly more fatigued than GenPop subjects (mean difference of 6.8, p < 0.001), but at baseline and at three, six, and 12 months after RT, no differences were found between patients and GenPop subjects (). Subgroup analyses defined by comorbidity revealed that this difference (at T2) was significant only among groups without comorbidity (mean 35.9 vs. 25.8, p < 0.001). Fatigue levels in patients with comorbidity did not differ significantly at any assessment point from the levels in GenPop subjects with comorbidity, and at T5 patients were slightly less fatigued than GenPop subjects (mean difference at 5.6).
Table V. Fatigue mean scores in BC patients during first year after RT compared with the general Norwegian population.
Discussion
The present longitudinal study investigated the one-year post-treatment course of fatigue in BC patients with local or locoregional RT, and with or without other adjuvant treatments. Furthermore, we explored the influence of comorbidity on fatigue levels, and finally we compared fatigue levels in patients with fatigue levels in the Norwegian GenPop.
Our finding of a temporary increase in fatigue during RT, followed by a prompt decline to pre-treatment levels after three months, is consistent with other studies [Citation7,Citation8]. Results from two recent studies indicated that a greater extent of RT might worsen fatigue during treatment [Citation9,Citation22]. In the one-year follow-up, neither the level nor the course of fatigue differed significantly by the extent of RT (local vs. locoregional) in analyses adjusted for CT, HT, and comorbidity.
CT was completed four to six weeks before starting RT in our study. We found no association between CT and overall fatigue, consistent with others [Citation7,Citation10–12,Citation18]. However, some evidence exists that CT-RT patients are more likely to be fatigued than RT patients [Citation13,Citation14,Citation17]. The significantly different course of fatigue in these groups was probably attributed to higher baseline levels among CT-RT patients [Citation9], since all assessments after the end of RT were almost identical, a pattern consistent with findings in a similarly designed study [Citation7]. In contradiction, a long-term study showed that the severity of fatigue increased from six to 42 months in CT-RT patients, whereas in RT patients levels remained stable or decreased [Citation14]. Contrasting results between different studies may partly be attributed to different CT-regimens with side effects of varying severity. Different lengths in follow-up periods may also lead to discrepancies in results when evaluating time patterns. Finally, the broad diversity of fatigue measures makes it challenging to perform confident comparisons between studies.
HT was not associated with overall fatigue in our sample, as reported by others [Citation10,Citation11,Citation18]. However, whereas mean level of fatigue did not differ according to extent of RT among patients receiving HT, it was a significant difference in patients without this treatment in our study. It is difficult to explain this finding, and results need to be interpreted with caution in view of the imprecise estimates.
Comorbidity emerged as the most important determinant of increased fatigue. BC patients with one or more chronic diseases (cardiovascular, pulmonary, diabetes, or depression) had generally elevated levels of fatigue, but this level remained stable during RT treatment. Hence, these patients may have adapted to higher fatigue levels, and perhaps altered their perception of symptom severity, i.e. response shift [Citation4,Citation8,Citation30]. Thus, RT may not be experienced as an extra burden for these patients, revealing comorbidity as a potential effect modifier of the associations with RT. Though the differences in levels of fatigue in patients with and without comorbidity were of moderate clinical significance, the pattern reveals that fatigue measured after BC treatment could be related to conditions other than the cancer or its treatment [Citation15,Citation16]. Our results highlight the importance of reliable assessments of comorbidities, as well as accounting for this underlying predictor of fatigue in both cross-sectional and longitudinal studies.
Surprisingly, we observed no significant differences in fatigue between BC patients and GenPop subjects, except for immediately after ending RT. Our results are promising if the pattern is attributed to a lessening in side effects due to advancements in modern treatments. However, going through a cancer treatment may change the perception of what “severe fatigue” represents, hence response shift in patients could be one part of the explanation [Citation30]. Consistent with our findings, another Scandinavian survey found no difference in vitality between BC survivors and GenPop subjects [Citation21]. Cross-sectional studies assessing fatigue with the EORTC questionnaire have reported significantly higher levels of fatigue in BC patients than GenPop subjects [Citation3,Citation18]. However, the inclusion of patients with distant metastasis [Citation3] and assessments at different times following treatment [Citation3,Citation18] complicates the generalizability of results to the BC survivor population and to specific times post-treatment. There are also substantial differences in EORTC-fatigue scores among GenPop subjects in different countries and Norwegians’ score at the upper layer (the most fatigued) [Citation29]. Hence, different reference scales may lead to variations in results between countries.
A major strength of our study is the high compliance rate and rather large sample size. Our study performed frequent follow-up assessments during the first year after treatment, including baseline assessments before starting RT. The unidimensional measure of fatigue could restrain the interpretations as it mostly covers the physical dimension [Citation27]. However, as the present study is part of a larger project, the scale’s brevity and ease of administration may have resulted in improved compliance. Furthermore, its widespread use expands the framework for interpretations [Citation26]. We measured fatigue within a relatively short timeframe after the completion of RT, and extended follow-up is needed. Data from GenPop was attained from a previous cross-sectional survey, and was not assessed in the same calendar period as our BC patients. However, the stability of the fatigue scale in the Norwegian GenPop, has been more lately confirmed [Citation16].
In summary, overall levels of fatigue were not significantly influenced by the extent of RT, by pre-treatment with CT, or by additional HT during the first year after RT in BC patients. The course of fatigue differed between CT-RT and RT patients during RT, but following the end of RT, and at all assessments in the subsequent year, equal levels were evident. Except for immediately after ending RT, BC patients were not significantly more fatigued than a sample from the Norwegian GenPop. Comorbidity emerged as the greatest determinant of increased fatigue, which highlights the importance of assessing chronic conditions when evaluating fatigue after cancer treatment. Prevention or treatment of comorbidities might be considered in rehabilitation programs of BC survivors.
http://informahealthcare.com/doi/abs/10.3109/0284186X.2012.742563
Download PDF (246.5 KB)Acknowledgements
The authors would like to thank the radiotherapy technicians, physicians, and nurses at the Department of Oncology at St. Olavs University Hospital for assisting with data collection, as well all patients participating in this study.
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
This research was supported by grants from Sør-Trøndelag University College and The Norwegian Women’s Public Health Association.
References
- Moulder S, Hortobagyi GN. Advances in the treatment of breast cancer. Clin Pharmacol Ther 2008;83:26–36.
- Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat 2008;112:5–13.
- Hoyer M, Johansson B, Nordin K, Bergkvist L, Ahlgren J, Lidin-Lindqvist A, . Health-related quality of life among women with breast cancer – a population-based study. Acta Oncol 2011;50:1015–26.
- Prue G, Rankin J, Allen J, Gracey J, Cramp F. Cancer-related fatigue: A critical appraisal. Eur J Cancer 2006;42:846–63.
- Groenvold M, Petersen M, Idler E, Bjorner J, Fayers P, Mouridsen H. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat 2007;105:209–19.
- Fan HG, Houede-Tchen N, Yi QL, Chemerynsky I, Downie FP, Sabate K, . Fatigue, menopausal symptoms, and cognitive function in women after adjuvant chemotherapy for breast cancer: 1- and 2-year follow-up of a prospective controlled study. J Clin Oncol 2005;23:8025–32.
- Noal S, Levy C, Hardouin A, Rieux C, Heutte N, Ségura C, . One-year longitudinal study of fatigue, cognitive functions, and quality of life after adjuvant radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2011;81:795–803.
- Donovan KA, Jacobsen PB, Andrykowski MA, Winters EM, Balducci L, Malik U, . Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. J Pain Symptom Manage 2004;28:373–80.
- Reidunsdatter RJ, Rannestad T, Frengen J, Frykholm G, Lundgren S. Early effects of contemporary breast radiation on health-related quality of life – Predictors of radiotherapy-related fatigue. Acta Oncol 2011;50:1175–82.
- Reinertsen KV, Cvancarova M, Loge JH, Edvardsen H, Wist E, Fossa SD. Predictors and course of chronic fatigue in long-term breast cancer survivors. J Cancer Surviv 2010; 4:405–14.
- Servaes P, Gielissen MF, Verhagen S, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: A longitudinal study. Psychooncology 2007;16:787–95.
- Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer 2010;116: 5740–8.
- Jacobsen PB, Donovan KA, Small BJ, Jim HS, Munster PN, Andrykowski MA. Fatigue after treatment for early stage breast cancer: A controlled comparison. Cancer 2007; 110:1851–9.
- Goedendorp MM, Andrykowski MA, Donovan KA, Jim HS, Phillips KM, Small BJ, . Prolonged impact of chemotherapy on fatigue in breast cancer survivors. Cancer 2012; 118:3833–41.
- Alonso J, Ferrer M, Gandek B, Ware JE, Jr., Aaronson NK, Mosconi P, . Health-related quality of life associated with chronic conditions in eight countries: Results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res 2004;13:283–98.
- Fossa SD, Hess SL, Dahl AA, Hjermstad MJ, Veenstra M. Stability of health-related quality of life in the Norwegian general population and impact of chronic morbidity in individuals with and without a cancer diagnosis. Acta Oncol 2007;46:452–61.
- Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, . Fatigue in long-term breast carcinoma survivors: A longitudinal investigation. Cancer 2006;106:751–8.
- Ahn SH, Park BW, Noh DY, Nam SJ, Lee ES, Lee MK, . Health-related quality of life in disease-free survivors of breast cancer with the general population. Ann Oncol 2007; 18:173–82.
- Hjermstad MJ, Fayers PM, Bjordal K, Kaasa S. Health-related quality of life in the general Norwegian population assessed by the European Organization for Research and Treatment of Cancer Core Quality-of-Life Questionnaire: The QLQ = C30 (+ 3). J Clin Oncol 1998; 16:1188–96.
- Klein D, Mercier M, Abeilard E, Puyraveau M, Danzon A, Dalstein V, . Long-term quality of life after breast cancer: A French registry-based controlled study. Breast Cancer Res Treat 2011;129:125–34.
- Peuckmann V, Ekholm O, Rasmussen NK, Moller S, Groenvold M, Christiansen P, . Health-related quality of life in long-term breast cancer survivors: Nationwide survey in Denmark. Breast Cancer Res Treat 2007;104:39–46.
- Taunk NK, Haffty BG, Chen S, Khan AJ, Nelson C, Pierce D, . Comparison of radiation-induced fatigue across 3 different radiotherapeutic methods for early stage breast cancer. Cancer 2011;117:4116–24.
- Wratten C, Kilmurray J, Nash S, Seldon M, Hamilton CS, O’Brien PC, . Fatigue during breast radiotherapy and its relationship to biological factors. Int J Radiat Oncol Biol Phys 2004;59:160–7.
- NBCG. Norwegian Breast Cancer Group. [cited 14 Nov 2012]. Available from: http://www.nbcg.no/index.html 2012.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Minton O, Stone P. A systematic review of the scales used for the measurement of cancer-related fatigue (CRF). Ann Oncol 2009;20:17–25.
- Knobel H, Loge JH, Brenne E, Fayers P, Hjermstad MJ, Kaasa S. The validity of EORTC QLQ-C30 fatigue scale in advanced cancer patients and cancer survivors. Palliat Med 2003;17:664–672.
- Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139–44.
- Twisk JWR. Applied longitudinal data analysis for epidemiology: a practical guide. Cambridge, UK; New York: Cambridge University Press; 2003.
- Andrykowski MA, Donovan KA, Jacobsen PB. Magnitude and correlates of response shift in fatigue ratings in women undergoing adjuvant therapy for breast cancer. J Pain Symptom Manage 2009;37:341–51.
- Fayers PM. Interpreting quality of life data: Population-based reference data for the EORTC QLQ-C30. Eur J Cancer 2001;37:1331–4.