Abstract
Endocrine therapy (ET) is a common method of treatment in breast cancer patients; however, its negative impact on body composition, body physique (physical body shape/measurements), and quality of life (QoL) remains controversial. Previous studies have shown physical exercise can have a positive effect on QoL in breast cancer patients, especially premenopausal subjects. Objective. In this feasibility study, we sought to assess the impact that physical exercise had on body composition and QoL in premenopausal breast cancer patients undergoing ET, and to determine the appropriateness of further testing of this intervention in this patient group. Material and methods. This study involved 41 premenopausal female breast cancer patients before and after six, 12, and 18 months of ET. Aerobic training began in the 6th month and resistance training was added in the 12th month. Body composition was evaluated using dual-energy x-ray absorptiometry (DXA) scans, body physique was evaluated using anthropometric measurement techniques, and QoL was evaluated using questionnaires from the European Organization for Research and Treatment of Cancer. Results. The initial period of ET with no exercise resulted in a reduction in fat-free body mass (FFBM), an increase in fat body mass (FBM), and a decline in QoL scores. Adding aerobic training resulted in a reduction of FBM and percentage of android fat, and improved QoL scores. The introduction of resistance training further reduced percentage of android and gynoid fat, increased FFBM, and further improved QoL scores. Conclusion. ET negatively impacts body composition, body physique, and QoL of premenopausal breast cancer patients. This feasibility study shows that physical activity may improve QoL and reduce adverse effects of ET on body composition and body physique, indicating appropriateness for further investigation on the use of exercise programs in premenopausal breast cancer patients to improve the outcomes of therapy.
Endocrine therapy (ET) is a common method of treatment in breast cancer patients with estrogen receptor expression, regardless of age and menopausal state [Citation1,Citation2]. However, it causes a range of negative effects such as osteoporosis, metabolic changes, and a decrease in quality of life (QoL) [Citation3,Citation4]. The deficiency in sex hormones induced in premenopausal women can result in psycho-emotional disorders, while patients can also experience a decrease in energy and motivation, changes in mood, anxiety, difficulty concentrating, and a decrease in libido [Citation4,Citation5]. ET in breast cancer patients can also have a negative influence on body composition and physique, which was presented in study by Francini et al. [Citation6]. Physical exercise can contribute to the outcomes of medical treatment, it is advisable in the prevention of many disorders, including cancer, and has been shown to have positive effects on energy and mood [Citation7–9]. An improvement in QoL due to regular exercise training is common in both healthy and sick individuals [Citation10,Citation11], with considerable research existing concerning the impact of physical activity on QoL in breast cancer patients [Citation8,Citation12], especially in postmenopausal women. In this feasibility study, we sought to assess the impact that physical exercise had on body composition and QoL in premenopausal patients undergoing ET, and to determine the appropriateness of further testing of this intervention in such cases.
Material and methods
Setting and participants
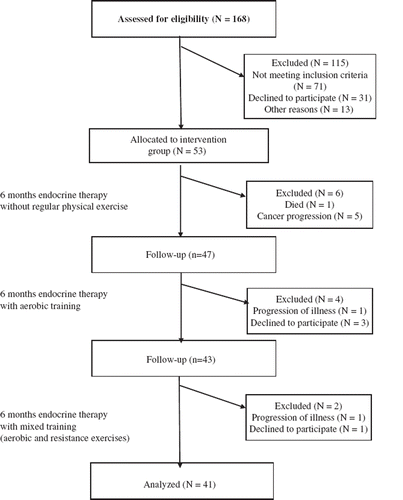
The study was conducted at the Department of Rehabilitation in the Greater Poland Cancer Centre in Poznan between September 2007 and December 2010. All study procedures were reviewed and approved by the Bioethics Committee at Poznan University of Medical Sciences, and participation in the study was conditioned by obtaining a voluntary informed consent. Fifty-three women receiving breast cancer treatment were initially selected for the study, with 41 women completing the program (study discontinuation in 12 subjects was caused by death, initiation of additional therapy due to disease progression, and failure to adhere to the required exercise protocols). Eligibility criteria included female breast cancer patients between 18 and 50 years old, premenopausal, i.e. regular menstruation until the introduction of ET, scheduled hormonal treatment planned to continue for a minimum of 18 months (goserelin 3.6 mg every 28 days and tamoxifen 20 mg daily), no distant metastases, no endocrinological, rheumatic, or cardiac diseases resulting in circulation failure (above Stage II of Heart Failure according to the New York Heart Association), no absorption disorders, no other tumors, and good general health (ECOG performance status 0-1). To make the study population more homogenous we included only patients undergoing goserelin and tamoxifen therapy as these are the most common ET drugs for premenopausal women in Poland. The study involved all eligible patients treated with those two drugs at our center between September 2007 and May 2009. Exclusion criteria were distant metastases and/or disease progression resulting in radiotherapy or the introduction of chemotherapy, withdrawal from the study before the 18-month period, or death of a patient during the course of the study. The study protocol was described in accordance with the CONSORT statement ().
Assessment schedule
All study participants were also involved in a study investigating bone density changes as a result of ET, and all examination periods ran concurrently with those predetermined for the bone evaluations. In the classified group of patients, the following schedule was used: Assessment I (Baseline) before the ET onset, Assessment II after six months of ET, Assessment III after six months of aerobic training and 12 months of ET, and Assessment IV (Final Assessment) after six months of resistance training, 12 months of aerobic activity, and 18 months of ET.
Assessment
Body physique measurements.
Body physique was assessed using anthropometrical parameters such as height, weight, waist circumference, and hip circumference. These measurements were used to determine the body mass index (BMI) and waist to hip ratio (WHR).
Body composition measurements.
Body composition was evaluated using dual-energy x-ray absorptiometry (DXA) scans (Lunar Prodigy Advance (GE, Madison, WI, USA) with the software enCORE (GE Healthcare V. 10.50.086) calibrated daily), all of which were evaluated by the same radiologist. The data calculated from the scans included fat body mass (FBM), fat-free body mass (FFBM), lean body mass (LBM), percentage of total body fat (%TBF), percentage of android fat (%AF), and the percentage of gynoid fat (%GF).
Quality of life measurements.
Quality of life was evaluated using the European Organization for Research and Treatment of Cancer (EORTC) questionnaire, QLQ-C30 version 3.0 and the breast cancer specific module QLQ-BR23. QLQ-C30 is a self-administered questionnaire that is specifically designed for the evaluation of QoL in cancer patients during clinical trials and the QLQ BR23 is a supplementary measure for use among breast cancer patients [Citation13]. The EORTC approved the use of these questionnaires in this study.
Exercise program
The study exercise program began after six months of ET therapy with aerobic exercises in order to evaluate the effects of ET therapy prior to the initiation of exercise. The aerobic activities were completed either alone or in groups and took place at the Rehabilitation Ward in the Greater Poland Cancer Centre under the supervision of at least one physiotherapist.
Aerobic training.
Between the 6th and 12th month of ET, patients performed aerobic exercise daily in accordance with the study exercise program. The optional exercises included brisk walking, running outside or on a treadmill, and various cycling activities. All activities lasted approximately 40–45 min. The workout consisted of a 2-min warm-up and 40 min of one of the activities, followed by a 3-min relaxation period. The physical activity was moderate, with the maximal heart rate of 65–75% of the maximum heart rate (220–age).
Resistance training.
In the 12th month of the study patients began resistance exercises with the goal of increasing muscle mass. The patients carried on their daily aerobic workout as they did between the 6th and 12th month of the study, which was verified via patient activity diaries. Resistance exercises were prescribed three times a week for approximately 40–45 min. The weight training sessions were conducted with one to two sets of six to eight isometric exercises for the trunk muscles, three sets of upper body exercises with 10–15 repetitions, and a 2-s concentric – 1-s static – 2-s eccentric time under tension, intermittent with 30–40 s of continuous stretching using elastic resistance bands (Thera-Band; Hadamar, Germany). To ensure progressive development of strength, different elastic resistance bands (1 kg, 2.5 kg, 3.5 kg, 4.5 kg per 100% extension) were used. Additionally, two sets of three unilateral, dynamic, weight-bearing leg exercises were performed in a circuit mode, with 1 min of exercise and 1 min of rest, with combined stretching exercises for corresponding muscle groups. The participants were asked to perform 16 repetitions per leg (2-s – 0-s – 2-s time under tension mode) on a submaximum exertion level. Intensity was progressively increased by enlarging the amplitude of the movement, changing the velocity of the concentric execution, and introducing more strenuous exercises. Every six weeks, new training with more intense exercises replaced the existing protocol. All the participants were advised not to take part in other forms of weight exercise during the study period, which was verified via patient interviews.
Exercise program adherence.
In the course of the study, some breaks from the exercises were allowed, which were to last no more than three days per month. Study organizers verified patients’ exercise program through physical activity notebooks that were checked by a physician in the rehabilitation department once a week.
Statistical analysis
The statistical data was analyzed using STATISTICA software (version 10.0 StatSoft Poland). The results of anthropometric measurements, DXA scans, and QoL questionnaires were analyzed. The quantitative data was described through mean and standard deviation. The Shapiro-Wilk's test was used to assess the normal distribution compatibility. The differences between the results were described using the t-test and Wilcoxon's test for connected variables, taking into account the size of the 95% confidence interval (CI). The results with p ≤ 0.05 were regarded as statistically significant and those with p ≤ 0.01 were regarded as highly statistically significant.
Results
The flow chart of inclusion and of study protocol is presented in and the participants’ characteristics and a summary of adherence to the study exercise program are presented in . The average days missed of aerobic training during the 12-month period was 15, with a minimum value of three days missed and a maximum value of 32 days missed. The average days of resistance training missed in the six-month period was five, with a minimum value of 0 and a maximum value of 11. Four participants were excluded from the study due to adherence problems: exercises were not being performed properly, there had been more than a 14-day break in adherence to the exercise program, or exercises were not being done the appropriate amount of times per week.
Table I. Characteristics of the study group.
summarizes the mean scores and changes in mean scores during the course of the study for body physique and composition, and QoL, respectively.
Table II. Mean and standard deviation values (SD) of BMI, WHR and body composition in separate stages of the study as well as the mean differences between the study periods.
Table III. Mean and standard deviation values (SD) of results from QLQ-C30 questionnaires at separate stages of the study, as well as the mean differences between the separate study periods.
Table IV. Mean and standard deviation values (SD) of results from QLQ-BR23 questionnaires at separate stages of the study, as well as the mean differences between the separate study periods.
Assessment I (Baseline) – prior to ET
Before ET, a statistically significant reverse relationship was found between emotional functioning and % gynoid fat. As for symptoms, directly proportional relationships were found between levels of fatigue, insomnia, and % gynoid fat (data not shown).
Assessment II – After six months of ET
Significant changes in body physique and composition were observed after six months of ET without regular physical activity, including elevated BMI, WHR, FBM, %TBF, and %AF (). Further, the patients reported an increase in role functioning but a reduction in physical functioning and an increase in symptom levels (e.g. fatigue), although they did experience less pain (). QLQ-BR23 results revealed a statistically significant betterment in the assessment of systemic therapy side effects and a decrease in breast symptoms ().
Assessment III – After 12 months of ET and six months of aerobic training
Six months of aerobic training resulted in significant reduction of FFBM and %AF () whereas there was an increase in overall QoL and a decrease in level of adverse symptoms (most notably fatigue and dyspnea) (), while the QLQ-BR23 ratings showed an increase in the functional scale levels and a decrease in levels of the symptoms scales ().
Assessment IV (Final Assessment) – After 18 months of ET, 12 months of aerobic training, and six months of resistance training
In the 18th month of the study, a reduction of body weight was noted, including a significant decrease in WHR, and a simultaneous increase in LBM and FFBM (). Analyses of QLQ-C30 showed a significant increase in overall QoL and functioning levels, and decreasing levels of adverse symptoms, especially fatigue, pain, and insomnia (). QLQ-BR23 showed better overall functioning, improved positive body image, and a reduction in symptom levels, especially systemic therapy side effects and breast symptoms ().
Discussion
In our study, we assessed the influence of physical activity on body physique, body composition, and QoL in premenopausal breast cancer patients undergoing ET (goserelin and tamoxifen). ET is known to cause deterioration of body physique, body composition, and overall QoL in premenopausal women. Regular exercise was shown to alleviate the negative results of this therapy.
During ET, the study group showed body physique and body composition changes, which have previously been described by other authors [Citation14]. These types of changes can cause metabolic disorders such as hypertension, diabetes, and metabolic syndrome, which can all be improved by physical exercise. The study group began with aerobic training, which reduced BMI after six months. The changes affected mostly waist to hip ratio and caused a decrease in fat body mass, percentage of total body fat, and especially percentage of android fat. Similar results were reported by Ohkawara et al. [Citation15] in obese cancer-free subjects without metabolic-related disorders. In the initial six months of aerobic training in this study, no regain of muscle mass was found and fat-free body mass was reduced (as it was prior to the initiation of exercise), which led study directors to implement resistance training after six months of aerobic exercise. During a pre-session, all study participants were instructed on the schedule, exercises, protocols, and requirements of the exercise program. The prescribed exercise duration, frequency, and intensity were matched to maximize possible results, as previous studies had shown less intensive and frequent exercises than prescribed in our study did not achieve desired results [Citation16]. Additionally, the American Cancer Society (ACS) recommends cancer patients in therapy should participate in exercise programs with higher frequency and intensity than recommended for the general public [Citation17]. Body physique and composition measurements in the assessment at month 18 (after 12 months of aerobic exercise and six months of resistance training) confirmed the changes in body assessments influenced by the resistance exercises that were described by other authors [Citation18–20], and give credence to the fact that exercise training has a positive impact on increasing lean body mass during hormonal changes. Similar results were published by Velthuis et al. [Citation18] in a randomized study involving 189 healthy postmenopausal women, who reported fat loss significant muscle gain in the DXA evaluation, induced by aerobic-resistance physical activity. Schmitz et al. [Citation19] confirmed that resistance exercises performed twice a week resulted in significant growth of muscle weight and lower fat content in 85 breast cancer survivors. Orsatti et al. [Citation20] studied a group of 43 healthy postmenopausal women and proved substantial muscle gain and fat loss after 16 weeks of the resistance training twice a week.
Hormonal deficiency affects physical, emotional, intellectual, and social functioning [Citation4,Citation5]. However, a decline in sexual enjoyment was also found, which may be connected with ET, stress induced by sickness, and a negative body image, resulting in the lack of self-attractiveness [Citation21]. The study by Montazeri et al. [Citation22] found lowered QoL, including physical functioning, whereas adverse symptoms such as fatigue, pain, and financial problems had sharply increased in time after primary treatment of 167 breast cancer women, mean age 47.2 years. In our study, we observed that reduced QoL corresponded with body physique and composition changes, and found an increase in adverse symptoms of ET correlating significantly with the percentage of android fat. Rehabilitation during oncological treatment greatly improves QoL at different stages of the oncological therapy [Citation13,Citation23]. Regular physical exercise may lead to a reduction in excessive body mass and mood improvement (lowering anxiety and depression and increasing the threshold of emotional stress). After the aerobic training, the overall QoL and physical functioning seemed to have improved, whereas a decline in adverse effects was found using the QLQ-C30 questionnaire and QLQ-BR23 module. We also observed that daily aerobic exercise were associated with beneficial changes on body composition and QoL, which confirmed the results reported by other authors presented in the meta-analysis of 22 randomized controlled trials involving breast cancer patients [Citation23]. After the implementation of combined training (aerobic and resistance) in the 18th month of the observation, further improvement of physical, emotional, social and role functioning was found. No relationship was found between self-reported symptoms and body composition (similar to the results found before ET began). The improvement of QoL may be due to a longer postsurgery recovery period, as well as the patients’ satisfaction from better physical fitness. Other authors’ studies also showed similar changes in the assessment of QoL under the influence of aerobic and weight training [Citation24,Citation25]. Milne et al. [Citation24] evaluated the effects of mixed (aerobic and resistance) exercises in a group of 58 breast cancer survivors and observed the QoL improvement measured by the Functional Assessment of Cancer Therapy-Breast Scale as well as in regard to fatigue, anxiety, and physical fitness. Rogers et al. [Citation25] investigated physical activity in 483 breast cancer patients using the International Physical Activity Questionnaire (IPAQ) and found significantly amended QoL, including fatigue and depressive symptoms. Our study indicates that physical activity can help prevent some of the negative effects of ET on body physique and body composition, while improving patient reported QoL and reducing the adverse effects of this adjuvant cancer therapy.
The limitations of this study include the lack of a control group, the relatively small number of participants, and a limited number of assessment periods (patients did not live close to the testing center and it was difficult for them to come in more frequently for assessments). However, the data from this small scale study indicated statistically significant changes in body physique, composition, and QoL, and substantiates further research on this topic. In future research, a prospective randomized controlled study including a physical and/or psychological intervention seems to be necessary to examine the effects of the interventions more thoroughly.
In our study we included only premenopausal women, whereas most studies in this area have included postmenopausal breast cancer patients. The most important aspect of this study include the novel observation that physical activity may play a therapeutic role in improving the quality of life and reducing the adverse effect of adjuvant endocrine treatment as well as improve physical fitness and body physique in premenopausal breast cancer patients.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Puhalla S, Brufsky A, Davidson N. Adjuvant endocrine therapy for premenopausal women with breast cancer. Breast 2009;18(Suppl 3):122–30.
- Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ, . Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol 2009;20:19–29.
- Angelopoulos N, Barbounis V, Livadas S, Kaltsas D, Tolis G. Effects of estrogen deprivation due to breast cancer treatment. Endocr Relat Cancer 2004;11:523–35.
- Bhattacharya SM. Health-related quality of life following surgical menopause and following gonadotrophin-releasing hormone analogue-induced pseudomenopause. Gynecol Endocrinol 2009;25:621–3.
- Biglia N, Moggio G, Peano E, Sgandurra P, Ponzone R, Nappi RE, . Effects of surgical and adjuvant therapies for breast cancer on sexuality, cognitive functions, and body weight. J Sex Med 2010;7:1891–900.
- Francini G, Petrioli R, Montagnani A, Cadirni A, Campagna S, Francini E, . Exemestane after tamoxifen as adjuvant hormonal therapy in postmenopausal women with breast cancer: Effects on body composition and lipids. Br J Cancer 2006;95:153–8.
- Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol 2011;50:187–93.
- Spence RR, Heesch KC, Brown WJ. Exercise and cancer rehabilitation: A systematic review. Cancer Treat Rev 2010;36:185–94.
- Hansen C, Stevens L, Coast R. Exercise duration and mood state: How much is enough to feel better?Health Psychol 2001;20:267–75.
- Peluso MA, Guerra de Andrade LH. Physical activity and mental health: The association between exercise and mood. Clinics 2005;60:61–70.
- Rogers LQ, Hopkins-Price P, Vicari S, Markwell S, Pamenter R, Courneya KS, . Physical activity and health outcomes three months after completing a physical activity behavior change intervention: Persistent and delayed effects. Cancer Epidemiol Biomarkers Prev 2009;18:1410–8.
- Chen X, Zheng Y, Zheng W, Gu K, Chen Z, Lu W, . The effect of regular exercise on quality of life among breast cancer survivors. Am J Epidemol 2009;170:854–62.
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, . The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–76.
- Thomson CA, Thompson PA, Wright-Bea J, Nardi E, Frey GR, Stopeck A. Metabolic syndrome and elevated C-reactive protein in breast cancer survivors on adjuvant hormone therapy. J Womens Health 2009;18:2041–7.
- Ohkawara K, Tanaka S, Miyachi M, Ishikawa-Takata K, Tabata I. A dose-response relation between aerobic exercise and visceral fat reduction: Systematic review of clinical trials. Int J Obes (Lond) 2007;31:1786–97.
- DeNysschen CA, Brown JK, Cho MH, Dodd MJ. Nutritional symptom and body composition outcomes of aerobic exercise in women with breast cancer. Clin Nurs Res 2011;20:29–46.
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, . Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:242–74.
- Velthuis MJ, Schuit AJ, Peeters PH, Monninkhof EM. Exercise program affects body composition but not weight in postmenopausal women. Menopause 2009;16:777–84.
- Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Can Epi Bio Prev 2005;14:1672–80.
- Orsatii FL, Nahas EA, Maesta N. Plasma hormones, muscle mass, strength in resistance-trained postmenopausal women. Mauritas 2008;59:394–404.
- Arndt V, Stegmaier C, Ziegler H, Brenner H. A population-based study of the impact of specific symptoms on quality of life in women with breast cancer 1 year after diagnosis. Cancer 2006;107:2496–503.
- Montazeri A, Vahdaninia M, Harirchi I, Ebrahimi M, Khaleghi F, Jarvandi S. Quality of life in patients with breast cancer before and after diagnosis: An eighteen months follow- up study. BMC Cancer 2008;11:330–2.
- Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, . Physical activity for cancer survivors: Meta-analysis of randomised controlled trials. BMJ 2012;344:e70.
- Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Can Res Treat 2008;108:279–88.
- Rogers LQ, Markwell SJ, Courneya KS, McAuley E, Verhulst S. Physical activity type and intensity among rural breast cancer survivors: Patterns and associations with fatigue and depressive symptoms. J Cancer Surviv 2011;5:54–61.