Abstract
Background. It has long been recognized that some human breast cancers are hormone dependent. Preeclampsia is a syndrome of pregnancy defined by the onset of hypertension and proteinuria and characterized by dysfunction of the maternal endothelium. Many hormonal changes occur with preeclampsia, and we hypothesize that these changes may influence the risk of maternal breast cancer. We also analyzed the relation between pregnancy-induced hypertension (PIH) and maternal risk of breast cancer. Methods. Among 13 relevant publications about preeclampsia and six relevant publications about PIH, some studies find preeclampsia associated with a lower risk of breast cancer, but others did not. Therefore, these results are inconclusive. We conducted meta-analysis to evaluate more precisely the relationship between preeclampsia, PIH and maternal risk of breast cancer. Results. The pooled estimate of the hazard ratio (HR) associated with preeclampsia was 0.86 (95% CI 0.73–1.01), and that associated with PIH was 0.83 (0.66–1.06), both based on the random effects model. Conclusion. Some suggestive but not entirely consistent nor conclusive evidence was found on the association between the history of preeclampsia or PIH with the subsequent risk of breast cancer.
Breast cancer is one of the hormone-dependent malignancies, and is especially influenced by estrogen. There are many conditions that change estrogen levels in women, such as pregnancy. For this reason, pregnancy is a critical time in relation to a woman's risk of breast cancer in later life [Citation1]. The mechanism through which pregnancy impacts breast cancer is still unclear, but many studies propose similar theories about hormonal changes during pregnancy [Citation2,Citation3]. Pregnancy characteristics known to influence the hormonal profile are multiple births (twin or higher order births), preeclampsia, pregnancy-induced hypertension (PIH), infant sex, gestational age (GA), fetal growth, pregnancy weight gain, gestational diabetes (GDM) and placental characteristics [Citation1]. Hormonal profiles including estrogen, progesterone, androgens, human chorionic gonadotropin (hCG), and insulin-like growth factor-I (IGF-I) are affected by pregnancy. Preeclampsia, a common complication of pregnancy, is a major cause of morbidity and mortality during pregnancy. Hypertension and proteinuria are hallmarks of preeclampsia, which is characterized by endometrial dysfunction. There are several mechanisms including the renin-angiotensin system, platelets, the role of androgens, the role of carbon monoxide, nitric oxide and prostacyclines responsible for the development of preeclampsia [Citation4,Citation5]. We focused on the role of androgens and their relationships with estrogen levels in preeclamptic conditions. There are some controversies about maternal serum estrogen and androgen concentrations in preeclamptic pregnancies [Citation6]. Lower estrogen concentrations and higher androgen concentrations observed in preeclampsia could be due to reduced aromatase activity, which would result in lower estrogen levels and higher circulating androgen levels [Citation4,Citation5,Citation7]. However, some studies showed lower estriol concentrations in preeclampsia [Citation8–11] while other studies showed no difference between preeclamptic and normal pregnancies [Citation12,Citation13]. We hypothesized that the elevation in estrogens levels during preeclamptic pregnancies increases the risk of maternal breast cancer development in later life. Based on this theory, we summarized published studies on this topic and discussed the results of a meta-analysis of published data on breast cancer risk in women who had preeclamptic pregnancies.
Material and methods
Search strategy
We searched literature using the National Library of Medicine (Pubmed) and the Cochrane Library to identify relevant articles. The following keywords and subject terms were used: ‘preeclampsia’, ‘pregnancy induced hypertension’, ‘gestational hypertension’ and ‘breast cancer’. The language of the papers was not restricted. References cited in the studies were reviewed to identify any additional studies that were not indexed by the electronic databases.
Data extraction
We followed a standard protocol for data extraction. For each study, we recorded the first author's name, year of publication, study design, number of cases of preeclamsia, PIH and normal pregnancies, the estimates of the hazard ratios and 95% confidence intervals in each group.
Statistical analysis
The Q-statistic was used to investigate the degree of heterogeneity between trials. The parameter to be estimated based on the pooled analysis is the hazard ratio. A p-value < 0.1 was interpreted as evidence of greater heterogeneity among the combined trials than would be expected by chance alone. The I2 measure was computed to describe the proportion of total variation caused by situations in which there were few studies or excessive power to detect clinically unimportant heterogeneity when there were many studies. I2 values of 25%, 50%, and 75% were used as evidence of low, moderate, and high heterogeneity, respectively. Assuming heterogeneity at the outset, we used a random effects model (DerSimonian-Laird method) to account for inter-study heterogeneity. All statistical analyses were carried out with STATA MP statistical software (version 10.1; Stata Corporation, College Station, TX, USA). All statistical tests were two-sided.
Results
Characteristics of enrolled studies
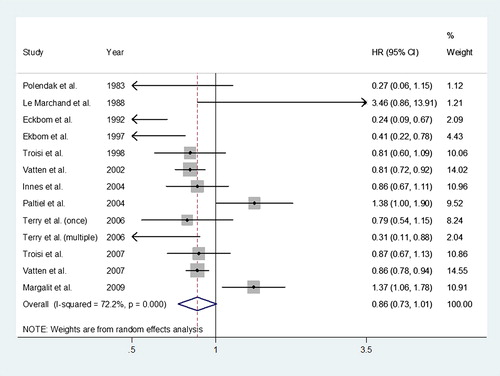
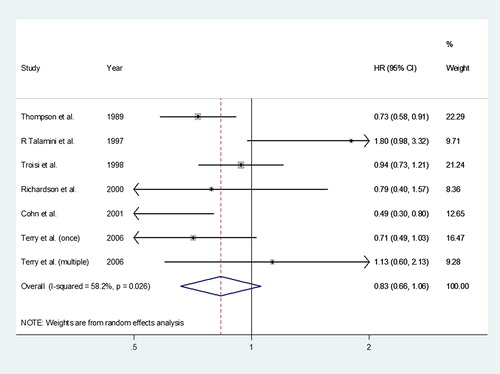
Eight case-control studies [Citation14–21] and five retrospective cohort studies [Citation22–26] were included. The 13 studies are described in and . All studies gave a hazard ratio (HR) and a 95% conference interval. The studies eligible for analysis were published between 1983 and 2009. The total breast cancer cases included 24 854 women, and most of these cases (9160 cases) were from Norway. In nine studies, the history of preeclamptic pregnancy was found to be associated with reduced breast cancer risk [Citation14,Citation16–18, Citation20,Citation21,Citation23,Citation25,Citation26]. The HRs ranged from 0.24 to 0.87. The other four studies reported a higher risk of breast cancer in those who had preeclampsia with HRs between 1.37 and 3.45 [Citation15,Citation19,Citation22,Citation24]. Clinically, there are some differences between preeclampsia and PIH. Preeclampsia is defined as new onset of hypertension and proteinuria during the second half of pregnancy [Citation27]. However, PIH is a more comprehensive term and it remains unclear if PIH and preeclampsia are separate conditions or represent opposite ends of a spectrum of a single disease entity [Citation4]. As preeclampsia and PIH may be different conditions, we separately analyzed cases of preeclampsia and PIH. Some studies were divided into two analyses of preeclampsia and PIH, while others analyzed PIH only. For the meta-analysis of the association with breast cancer risk and PIH, another six studies were included [Citation17,Citation19,Citation20,Citation28–30]. Only one was a cohort study [Citation28] and all others were case-control studies [Citation17,Citation19,Citation20,Citation29,Citation30]. The studies eligible for analysis were published between 1989 and 2006. In the US study (Thompson et al.), the history of preeclamptic pregnancy was found to be associated with lower breast cancer risk (HR = 0.73, 95% CI 0.59–0.92) [Citation30]. There were 10 134 breast cancer cases and the largest number (4668 cases) were enrolled in the study [Citation30]. Half of the six studies did not show a protective effect of PIH on breast cancer risk within a lifetime (range of HR: 1.07–1.8) and the other half showed a protective effect of preeclampsia on breast cancer (range of HR: 0.49–0.94).
Table I. Studies investigating the association between preeclampsia and risk of breast cancer.
Table II. Studies investigating the association between PIH and risk of breast cancer.
Preeclampsia and breast cancer risk
The pooled estimate of the hazard ratio (HR) of breast cancer associated with previous history of preeclampsia was 0.86 (95% CI 0.73–1.01). A forest plot of studies reporting preeclampsia and the risk of breast cancer is shown in . We used a random effects model to combine the risk estimates since the overall heterogeneity was high (I2 = 72.2%).
Pregnancy-induced hypertension (PIH) and breast cancer risk
The pooled estimate of the hazard ratio (HR) of breast cancer associated with previous history of PIH was 0.83 (95% CI 0.66–1.06). A forest plot of studies reporting preeclampsia and the risk of breast cancer is shown in . We used a random effects model to combine the risk estimates since the overall heterogeneity was moderate to high (I2 = 58.2%).
Publication bias
Begg's funnel plot and the Egger test were used to analyze publication bias. There was no obvious asymmetry in the shape of the funnel (data not shown). Egger's test for small-study effects did not suggest any evidence of publication bias for preeclampsia and PIH, respectively (p = 0.599 and p = 0.612).
Discussion
In this meta-analysis, based on eight case-control and five cohort studies, we found some suggestive but not entirely consistent nor conclusive evidence for women with a history of preeclampsia or PIH having a reduced risk of breast cancer. In theory, women with preeclampsia may show decreased estrogen levels. Lower estrogen concentrations in preeclampsia are closely related to higher circulating androgen levels in preeclampsia. Acromite et al. published a paper about elevated serum androgens, especially testosterone, in primigravid women with preeclampsia [Citation5]. They observed that the level of testosterone was significantly higher in women with preeclampsia than in normotensive women with similar body mass index, gestational age, and chronologic age. According to that and other studies, androgens have a hypertension-promoting effect through the renin-angiotensin system [Citation31] and characteristic hypertensive conditions are induced. In addition, circulating androgens undergo aromatic transformation to estrogens through the action of placental microsomal enzyme, cytochrome P450 protein, during normal pregnancy [Citation32]. However, placental dysfunction exists in preeclampsia, and therefore pregnancies complicated by hypertension have deficiencies in placental aromatization enzymes [Citation33]. Without effective placental enzyme activity, levels of androgens cannot decline and estrogen levels may remain lower than in normal pregnancies. Therefore, women with preeclampsia may have less exposure to estrogen than normally experienced during pregnancy. Estrogen exposure is thought to be a major risk factor for breast cancer. Other authors have investigated whether women have lower estrogen levels in preeclampsia but the research did not support their hypothesis [Citation34]. However, subsequent studies measuring serum estrogen from preeclamptic women have been controversial, with some studies showing lower estriol values in preeclampsia [Citation8–11] while others show either no difference [Citation12,Citation13] or higher concentrations [Citation35]. Another possible mechanism of hormone effects on breast cancer in preeclampsia is through alpha-fetoprotein (AFP). Waller revealed a positive association between preeclampsia and placental complications and serum AFP level [Citation36]. Raty also studied predictors of preeclampsia and found that high mid-trimester serum AFP values may help in the prediction of severe preeclampsia [Citation37]. AFP, a protein product of fetal livers, is known to have an anti-hormonal effect. Therefore, we hypothesize that this anti-hormonal effect may reduce the effect of estrogen on the mammary glands and consequentially diminish breast cancer risk. Supporting this hypothesis, many studies have shown that AFP reacts with estradiol to generate a potent inhibitor of estrogen-sensitive cell response to estrogens [Citation38,Citation39]. In addition to these hormones, many studies have evaluated hormonal change in preeclampsia. Leptin and inhibin A may be related to sex hormones, but this hypothesis is still controversial [Citation37,Citation40].
Our meta-analysis has some limitations. First, there are differences between preeclampsia and PIH. Due to similar characteristics between the diseases, distinction of preeclampsia from PIH might be difficult. Misclassification of patients may be possible, especially in self-reported types of studies. Second, the patients who enrolled in each study could overlap; studies from the same country over different periods are enrolled in this meta-analysis. Third, all studies were performed only in Europe and US. They did not report data regarding the population origins of patients, but characteristics of Asian breast cancer patients are not included.
In our study, some suggestive but not entirely consistent nor conclusive evidence was found on the association between the history of preeclampsia or PIH with the subsequent risk of breast cancer.
Many complex hormonal changes occur during pregnancy. In complicated conditions like preeclampsia or PIH, these hormonal characteristics are very different. This complexity makes it difficult to interpret the pathogenesis of preeclampsia. Further studies will be needed to evaluate the effect of different factors on maternal breast cancer risk and prognosis, such as population, genetics, treatment response, disease severity, to reach meaningful conclusions.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by a grant of the Korean Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A040151).
References
- Nechuta S, Paneth N, Velie EM. Pregnancy characteristics and maternal breast cancer risk: A review of the epidemiologic literature. Cancer Causes Control 2010;21:967–89.
- Russo J, Russo IH. Toward a physiological approach to breast cancer prevention. Cancer Epidemiol Biomarkers Prev 1994;3:353–64.
- Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer 2006;6:281–91.
- Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur J Obstet Gynecol Reprod Biol 2006;126:16–9.
- Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet Gynecol 1999;180:60–3.
- Innes KE, Byers TE. Preeclampsia and breast cancer risk. Epidemiology 1999;10:722–32.
- Rosing U, Carlstrom K. Serum levels of unconjugated and total oestrogens and dehydroepiandrosterone, progesterone and urinary oestriol excretion in pre-eclampsia. Gynecol Obstet Invest 1984;18:199–205.
- Isouard G. Measurement of serum levels of oestriol and human placental lactogen in the management of pre-eclamptic pregnancies. Med J Aust 1979;2:401–4.
- Klopper A, Jandial V, Wilson G. Plasma steroid assay in the assessment of foetoplacental function. J Steroid Biochem 1975;6:651–6.
- Stamilio DM, Sehdev HM, Morgan MA, Propert K, Macones GA. Can antenatal clinical and biochemical markers predict the development of severe preeclampsia?Am J Obstet Gynecol 2000;182:589–94.
- Warren WB, Gurewitsch ED, Goland RS. Corticotropin- releasing hormone and pituitary-adrenal hormones in pregnancies complicated by chronic hypertension. Am J Obstet Gynecol 1995;172:661–6.
- Masse J, Forest JC, Moutquin JM, Marcoux S, Brideau NA, Belanger M. A prospective study of several potential biologic markers for early prediction of the development of preeclampsia. Am J Obstet Gynecol 1993;169:501–8.
- Ranta T, Stenman UH, Unnerus HA, Rossi J, Seppala M. Maternal plasma prolactin levels in preeclampsia. Obstet Gynecol 1980;55:428–30.
- Ekbom A, Trichopoulos D, Adami HO, Hsieh CC, Lan SJ. Evidence of prenatal influences on breast cancer risk. Lancet 1992;340:1015–8.
- Le Marchand L, Kolonel LN, Myers BC, Mi MP. Birth characteristics of premenopausal women with breast cancer. Br J Cancer 1988;57:437–9.
- Polednak AP, Janerich DT. Characteristics of first pregnancy in relation to early breast cancer. A case-control study. J Reprod Med 1983;28:314–8.
- Terry MB, Perrin M, Salafia CM, Zhang FF, Neugut AI, Teitelbaum SL, et al. Preeclampsia, pregnancy-related hypertension, and breast cancer risk. Am J Epidemiol 2007; 165:1007–14.
- Innes KE, Byers TE. First pregnancy characteristics and subsequent breast cancer risk among young women. Int J Cancer 2004;112:306–11.
- Richardson BE, Peck JD, Wormuth JK. Mean arterial pressure, pregnancy-induced hypertension, and preeclampsia: Evaluation as independent risk factors and as surrogates for high maternal serum alpha-fetoprotein in estimating breast cancer risk. Cancer Epidemiol Biomarkers Prev 2000;9: 1349–55.
- Troisi R, Weiss HA, Hoover RN, Potischman N, Swanson CA, Brogan DR, et al. Pregnancy characteristics and maternal risk of breast cancer. Epidemiology 1998;9:641–7.
- Troisi R, Innes KE, Roberts JM, Hoover RN. Preeclampsia and maternal breast cancer risk by offspring gender: Do elevated androgen concentrations play a role?Br J Cancer 2007;97:688–90.
- Calderon-Margalit R, Friedlander Y, Yanetz R, Deutsch L, Perrin MC, Kleinhaus K, et al. Preeclampsia and subsequent risk of cancer: Update from the Jerusalem Perinatal Study. Am J Obstet Gynecol 2009;200:63e1–5.
- Ekbom A, Hsieh CC, Lipworth L, Adami HQ, Trichopoulos D. Intrauterine environment and breast cancer risk in women: A population-based study. J Natl Cancer Inst 1997;89:71–6.
- Paltiel O, Friedlander Y, Tiram E, Barchana M, Xue X, Harlap S. Cancer after pre-eclampsia: Follow up of the Jerusalem perinatal study cohort. Br Med J 2004;328:919.
- Vatten LJ, Forman MR, Nilsen TI, Barrett JC, Romundstad PR. The negative association between pre-eclampsia and breast cancer risk may depend on the offspring’s gender. Br J Cancer 2007;96:1436–8.
- Vatten LJ, Romundstad PR, Trichopoulos D, Skjaerven R. Pre-eclampsia in pregnancy and subsequent risk for breast cancer. Br J Cancer 2002;87:971–3.
- Powe CE, Levine RJ, Karumanchi SA. Preeclampsia, a disease of the maternal endothelium: The role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 2011;123:2856–69.
- Cohn BA, Cirillo PM, Christianson RE, van den Berg BJ, Siiteri PK. Placental characteristics and reduced risk of maternal breast cancer. J Natl Cancer Inst 2001;93: 1133–40.
- Talamini R, Franceschi S, Favero A, Negri E, Parazzini F, La Vecchia C. Selected medical conditions and risk of breast cancer. Br J Cancer 1997;75:1699–703.
- Thompson WD, Jacobson HI, Negrini B, Janerich DT. Hypertension, pregnancy, and risk of breast cancer. J Natl Cancer Inst 1989;81:1571–4.
- Bachmann J, Feldmer M, Ganten U, Stock G, Ganten D. Sexual dimorphism of blood pressure: Possible role of the renin-angiotensin system. J Steroid Biochem Mol Biol 1991;40:511–5.
- Gant NF, Hutchinson HT, Siiteri PK, MacDonald PC. Study of the metabolic clearance rate of dehydroisoandrosterone sulfate in pregnancy. Am J Obstet Gynecol 1971;111:555–63.
- Hahnel ME, Martin JD, Michael CA, Hahnel R. Metabolism of androstenedione by placental microsomes in pregnancy hypertension. Clin Chim Acta 1989;181:103–8.
- Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, et al. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol 2003;32:455–60.
- Hughes G, Bischof P, Wilson G, Smith R, Klopper A. Tests of fetal wellbeing in the third trimester of pregnancy. Br J Obstet Gynaecol 1980;87:650–6.
- Waller DK, Lustig LS, Cunningham GC, Feuchtbaum LB, Hook EB. The association between maternal serum alpha-fetoprotein and preterm birth, small for gestational age infants, preeclampsia, and placental complications. Obstet Gynecol 1996;88:816–22.
- Raty R, Koskinen P, Alanen A, Irjala K, Matinlauri I, Ekblad U. Prediction of pre-eclampsia with maternal mid-trimester total renin, inhibin A, AFP and free beta-hCG levels. Prenat Diagn 1999;19:122–7.
- Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995;270:1491–4.
- Mizejewski GJ, Vonnegut M, Jacobson HI. Estradiol-activated alpha-fetoprotein suppresses the uterotropic response to estrogens. Proc Natl Acad Sci U S A 1983;80:2733–7.
- Atamer Y, Erden AC, Demir B, Kocyigit Y, Atamer A. The relationship between plasma levels of leptin and androgen in healthy and preeclamptic pregnant women. Acta Obstet Gynecol Scand 2004;83:425–30.