To the Editor,
Interstitial pneumonitis is a known, but uncommon adverse event during docetaxel therapy in cancer patients. We report the case of a patient with breast cancer who developed a severe, progressive pneumonitis during dose-dense neoadjuvant chemotherapy with docetaxel. The condition was steroid resistant, and she died after 20 days on advanced ventilator therapy in the intensive care unit. The pneumonitis was complicated by reduced lung compliance, bilateral pneumothorax and severe hypercapnia and hypoxia. To our knowledge, this is the first case of taxane-induced pneumonitis with a following autopsy report. We review the present literature about the condition and discuss potential disease mechanisms.
Case report
A previously healthy 58-year-old female was diagnosed with a locally advanced right-sided breast cancer in March 2012. Staging with ultrasound of regional lymph nodes, computed tomography (CT) of the chest and abdomen and whole body skeletal scintigraphy showed multiple lymph node metastases in the right axilla, but no distant metastasis. The cancer was classified as cT3 (7.7 cm) N1M0, infiltrating lobular carcinoma, estrogen receptor positive (80%), progesterone receptor positive (10%), HER2 not amplified, and Ki67 43%. After informed consent, she was included in a single-arm phase 2 trial of dose-dense neoadjuvant epirubicin followed by docetaxel (Clinical Trials.gov Identifier: NCT00496795 and Regional ethics committee approval: #079.06). She received four uneventful courses of i.v. epirubicin (60 mg/m2) q2w, followed by four courses of i.v. docetaxel (100 mg/m2) q2w. On a routine basis she received dexamethasone and ondansetrone before and after all chemotherapy courses, and additionally aprepitant before and after the epirubicin courses. Pegfilgrastim 6 mg s.c. was given 24 hours after all courses.
The epirubicin treatment was well-tolerated. Following the first and second docetaxel courses she experienced pain and a mild desquamation of the skin on her hands and feet, compatible with hand/foot syndrome, grade 2, according to the National Cancer Institute Common Terminology Criteria for Adverse events, version 3.0 (CTCAEv3.0) [Citation1]. Between docetaxel course three and four the C-reactive protein (CRP) increased from 21 to 83 mg/l. At the time of the fourth course she presented with a mild fever and dry cough, but vital signs were normal. The condition was interpreted as a minor respiratory tract infection, and she was started on oral phenoxymethylpenicillin, and the fourth docetaxel course was given concomitantly. The CRP decreased over the next four days to 53 mg/l, but the fever persisted around 38°C.
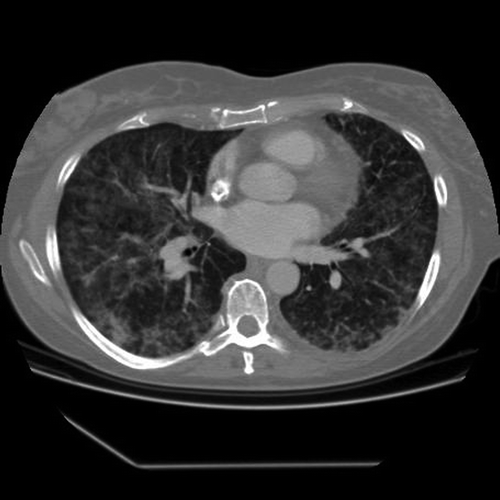
Eight days after the fourth docetaxel course she was admitted to hospital due to continuous fever 38.0°C, fatigue and a minor discomfort during deep inhalation. When admitted she had tachycardia and the CRP had increased to 99 mg/l. She was started on oral azithromycin on suspicion of atypical pneumonia, and chest x-ray and CT the following day () demonstrated bilateral interstitial densifications, interpreted by the radiologist as an atypical pneumonia. A wide panel of serological tests, blood cultures and nasal aspirates did not detect any infections, including negative findings for Mycoplasma pneumoniae, Chlamydia trachomatis, Bordetella pertussis, Cytomegalovirus and Influenzavirus A and B. A bronchoalveolar lavage (BAL) test for Pneumocystis jirovecii was not undertaken at this point, since the patient was found too dyspneic for bronchoscopy. Ten days after the last docetaxel injection, oral trimethoprim/sulfamethoxazole and predniolone 40 mg BID were added to the azithromycin treatment due to increasing respiratory distress. Despite these measures, her condition worsened progressively over the next three days, with substantial inhalation difficulties and fever up to 39.5°C. The systolic blood pressure (BP) decreased to 90 mm Hg and peripheral oxygen saturation fell to 89%, despite increasing oxygen supplementation from 1 to 10 l/min on face mask.
Figure 1. Chest CT scan 9 days after the fourth docetaxel course with widespread interstitial infiltrates.
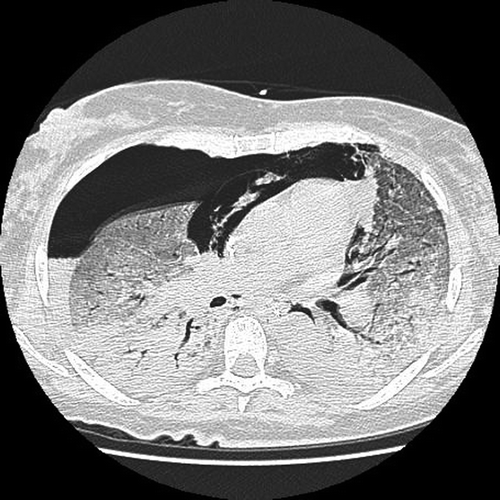
A new chest x-ray, 13 days after the last docetaxel injection, showed widespread and increasing interstitial infiltrates, and i.v. cefotaxime was added to her antibiotics. After a short attempt with mask-assisted continuous positive airway pressure (CPAP) treatment, the oxygen saturation decreased to 70% on FiO2 1.0, and she was moved to the intensive care unit (ICU) where she was intubated and ventilated, using Airway Pressure Release Ventilation (APRV, mean pressure 24 cm H2O). During the first day in the ICU a BAL test was performed, but P. jirovecii was not detected by polymerase chain reaction (PCR), and since no clinical improvement had been noted, antibiotic therapy was halted. In all subsequent tests neither bacteria nor virus were detected. On the sixth day in the ICU i.v. ciprofloxacine, meropeneme, and fluconazole were initiated due to fever and increasing CRP to 122 mg/l. On further suspicion of drug-induced pneumonitis she received i.v. dexamethasone 12 mg daily during the whole ICU stay. The lung compliance decreased rapidly, and the oxygen demand increased progressively. Despite a particular care to avoid high positive pressures in the airways during ventilator support, the patient developed bilateral pneumothorax, pneumomediastinum, and pneumopericardium (), and emergency insertion of bilateral chest drains was performed. Due to increasing hypoxia and hypercapnia, oscillator treatment, prone positioning, an Interventional Lung Assist (ILA) device and nitric oxide treatment were temporarily implemented, but without any respiratory improvement. Furthermore, the ILA treatment was complicated by repeated pleural bleeding due to the need for heparinization. All active treatment was ultimately withdrawn due to the continuing hypoxia, hypercapnia, bilateral pleural bleeding and persisting poor lung compliance. Lung transplantation was found to be contraindicated due to active cancer, and after 20 days on ventilator and 23 days on steroids the patient died with a single organ lung failure within 15 minutes of ventilator shutdown.
Figure 2. Chest CT scan 16 days after the fourth docetaxel course with severe interstitial pneumonitis, complicated by right-sided pneumothorax and pneumopericardium.
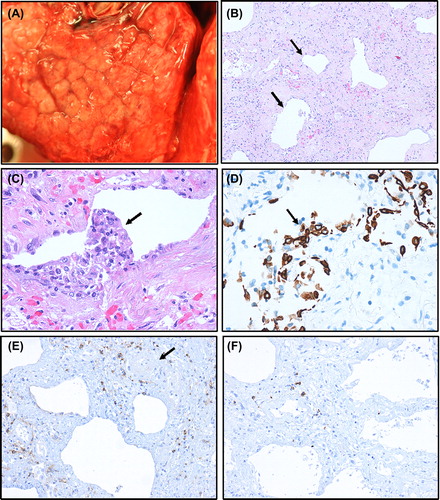
The autopsy demonstrated heavy and stiff lungs with a cobble-stoned appearance on the surface, delineating the alveolar septae (). Multiple air-filled bullae were seen on the lung surface. By microscopy the lung tissue presented as ‘end-stage lungs’ with extensive fibrosis and few remaining alveolar sacs, giving a honeycomb picture (), probably as a result of earlier diffuse alveolar damage in the acute phase from where sections are not available. Inflammation was scant and probably not representative for the acute phase. Only a few, scattered, perivascular T lymphocytes, some dispersed macrophages and mast cells were observed. Neutrophilic granulocytes were only found in bleeding areas, while eosinophils were not increased in number. P. jirovecii, other microorganisms or lymphangitis carcinomatosa were not found. Distant metastases from the primary lobular breast carcinoma were not found.
Figure 3. Photomicrographs (A–F) from lung tissue obtained at autopsy. Surface of lung with macroscopic cobblestone appearance evident (A). ‘End-stage’ lung tissue with severe interstitial fibrosis with a few remaining alveolar sacs (arrows) giving a honeycomb pattern (B). Due to postmortem autolysis most alveolar epithelium is detached, but in some alveolar sacs, the epithelium with regenerative atypia has remained as partly detached epithelial clusters (C, arrow), positively stained by the epithelial immunohistochemical marker cytokeratin (PAN) (D, arrow). Only a few scattered, mainly perivascular lymphocytes (arrow at vessel), are observed (E). A slight predominance by CD4 (E) over CD8 (F) T lymphocytes is observed. Magnification: × 100 (B), × 200 (E, F), × 400 (C, D).
Discussion and review of literature
Interstitial pneumonitis is a known, but rare complication of docetaxel chemotherapy [Citation2–4]. Docetaxel-induced pneumonitis was noted in the early phase 2 trials conducted in the mid-1990s [Citation4], but compared to other drug-induced cases of pneumonitis which typically last one to two weeks this was characterized by a much longer duration [Citation4]. According to previous reports pneumonitis is more commonly seen with paclitaxel than docetaxel [Citation3], and high-dose steroid administration with slow tapering is generally recommended [Citation3,Citation5,Citation6]. Before concluding that there is a case of drug-induced interstitial pneumonitis other causes, such as infection, lymphangitis carcinomatosa, other interstitial lung diseases, heart failure a.o. should be excluded [Citation6]. Infections and lung metastases were both excluded in our patient, her heart function was found normal by ultrasound examination, and she had no previous history of lung disease or autoimmune disease which could give similar lung findings.
To the best of our knowledge, this is the first report of a possible docetaxel-induced pneumonitis where a complete postmortem report is given. Few details are available on how to treat such patients in an optimal manner with regard to steroids, antibiotics and ventilator support. In previous cases a prolonged need for ventilator support, with certain late recoveries after two to three weeks in the ICU were reported [Citation3,Citation4]. When summarizing a total of 31 cases of taxane-induced pneumonitis, 35% needed ventilatory support, and whereas the mortality rate was 42% in the whole group, those in need of intubation had a mortality rate of 82% [Citation3].
Apart from taxanes, interstitial pneumonitis can be caused by many cancer drugs [Citation6,Citation7]. In the Federal Drug Agency (FDA, US) and European Medicines Agency (EMA) drug approval of filgrastim (Neupogen®, Amgen) and pegfilgrastim (Neulasta®, Amgen), pneumonitis is listed as an uncommon side effect, i.e. < 1/100 and > 1/1000 patients. While pegfilgrastim was administered after all chemotherapy courses, our patient did not develop dry cough, respiratory distress and fever until after the third course of docetaxel – indicating that docetaxel is the most probable cause.
Our department administers over 900 docetaxel courses every year and during the last 16 years since taxanes were introduced clinically in Norway, we have seen in our department only one mild, transient case of potential docetaxel pneumonitis in a patient with metastatic prostate cancer. One could speculate that pneumonitis in the current patient was related to the dose-dense regimen, but we have not observed similar cases in the 70 other patients included and treated in the trial thus far. Also, in a previous study of 38 patients receiving higher doses of epirubicin/cyclophosphamide (EC) × 6 followed by paclitaxel × 6 q2w, supported by pegfilgrastim, no cases of pneumonitis were reported [Citation8]. However, the authors state that a previous trial where 5-fluorouracil (5-FU) was added to EC, had to be stopped due to pneumonitis, and assumed it to be related to 5-FU [Citation8]. In another trial, 70 patients with HER2-amplified breast cancer were given adriamycin/cyclophosphamide (AC) × 4 followed by paclitaxel × 4 q2w with pegfilgrastim support, followed by trastuzumab for one year, with only one case of pneumonitis which occurred during the trastuzumab treatment [Citation9]. Using a similar chemotherapy regimen, two-weekly versus three-weekly courses were compared postoperatively in 2005 patients with node-positive breast cancer, and apart from improved disease-free and overall survival, pulmonary toxicity was not reported [Citation10]. Filgrastim and pegfilgrastim is commonly used to enable dose-dense chemotherapy in different chemotherapy regimens, but such G-CSF support stimulates the maturation of granulocytes and does not prevent lymphopenia [Citation11]. Thus, atypical pneumonia, caused by P. jirovecii, is a known, but infrequent complication during dose-dense chemotherapy, facilitated by the reduced circulating CD4+ lymphocyte numbers and immunosuppression due to supportive corticosteroid use [Citation12–14]. Our patient had a negative PCR test for P. jirovecii in the tracheal aspirate and no improvement on antibiotics, and it therefore seems unlikely that an atypical pneumonia was part of her worsening pneumonitis.
Taxanes are known to cause hypersensitivity reactions, commonly experienced as dyspnea or bronchospasm and indicating that the lungs are prone to taxane toxicity. Furthermore, docetaxel induces a fluid retention syndrome due to capillary leak [Citation15]. The hypersensitivity reaction and fluid retention can be prevented or dampened with high doses of dexamethasone [Citation16]. In a previous report the lung biopsy suggested that docetaxel-induced pneumonitis might be a hypersensitivity pneumonitis, comparable to what is sometimes seen with methotrexate [Citation4]. Pulmonary interstitial infiltrates have been described radiologically within hours to a few days after paclitaxel infusion [Citation17,Citation18], although in most cases these infiltrates are detected one to two weeks after taxane administration [Citation4,Citation19,Citation20]. Apart from the exclusion of other etiologies, the Japan Respiratory Society guidelines for evaluation of drug-induced interstitial pneumonitis state that BAL typically shows lymphocytic alveolitis, increased total cell number, increased neutrophils and eosinophils, and a decreased CD4/CD8 ratio [Citation3]. Furthermore, transbronchial biopsies typically reveal edema and swelling of the alveolar septa, aggregation of alveolar macrophages and using a provocation test, drug-specific T cells can be confirmed, suggesting a T cell-mediated immune response [Citation3]. By analysis of BAL and transbronchial biopsies, a lung cancer patient with paclitaxel- induced pneumonitis had a 36% increased lymphocyte and 2% increased eosinophil number in the BAL fluid, whereas transbronchial biopsies showed a decreased CD4/CD8 ratio of 0.78 in addition to increased macrophage infiltrates [Citation19]. This is in contradiction to our observation of increased CD4+ lymphocyte infiltration, but our analysis was done postmortem in end-stage lungs. In another case the BAL fluid revealed 69% lymphocytes (CD4/CD8 ratio 1), 18% neutrophils, 12% eosinophils, and 1% macrophages [Citation5]. Altogether, there is a clear impression that there is an induced immune reaction in the lung parenchyma after taxane exposure.
Apart from steroids to dampen the inflammatory component of interstitial pneumonitis, and antibiotics if P. jirovecii is detected, no further therapeutic measures are known to affect this complication, besides stopping the causative drug. The infiltration of CD4+ T lymphocytes detected in this case report should be further investigated to identify potential chemokines that attract these cells or cytokines that stimulate fibroblast proliferation and collagen deposition in the later stages of pneumonitis development. Furthermore, biopsy assessments at earlier stages of the pneumonitis should be considered to improve our understanding of how it develops. Until we know more, the clinician should be aware of this side effect and strongly consider stopping taxane therapy and start steroid treatment at the earliest signs of interstitial pneumonitis.
Acknowledgments
We gratefully acknowledge the contribution made to science by the patients included in the dose-dense trial and to the family of this patient in particular for allowing us to publish the case report. HPE was supported in his breast cancer research by generous grants from the Norwegian Cancer Society, the Rieber Foundation, Bergen Medical Research Foundation and the Eckbo Legacy, Norway.
Declaration of interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. Ctcae v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–81.
- Min BD, Kang HW, Kim WT, Kim YJ, Yun SJ, Lee SC, et al. Docetaxel-induced fatal interstitial pneumonitis in a patient with castration-resistant prostate cancer. Korean J Urol 2012;53:371–4.
- Nagata S, Ueda N, Yoshida Y, Matsuda H, Maehara Y. Severe interstitial pneumonitis associated with the administration of taxanes. J Infect Chemother 2010;16:340–4.
- Read WL, Mortimer JE, Picus J. Severe interstitial pneumonitis associated with docetaxel administration. Cancer 2002; 94:847–53.
- Merad M, Le Cesne A, Baldeyrou P, Mesurolle B, Le Chevalier T. Docetaxel and interstitial pulmonary injury. Ann Oncol 1997;8:191–4.
- Muller NL, White DA, Jiang H, Gemma A. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer 2004;91(Suppl 2):S24–30.
- Rossi SE, Erasmus JJ, McAdams HP, Sporn TA, Goodman PC. Pulmonary drug toxicity: Radiologic and pathologic manifestations. Radiographics 2000;20: 1245–59.
- Dang C, D’Andrea G, Lake D, Sugarman S, Fornier M, Moynahan ME, et al. Prolonged dose-dense epirubicin and cyclophosphamide followed by paclitaxel in breast cancer is feasible. Clin Breast Cancer 2008;8:418–24.
- Dang C, Fornier M, Sugarman S, Troso-Sandoval T, Lake D, D’Andrea G, et al. The safety of dose-dense doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab in her-2/neu overexpressed/amplified breast cancer. J Clin Oncol 2008;26:1216–22.
- Citron ML, Berry DA, Cirrincione C, Hudis C, Winer EP, Gradishar WJ, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of intergroup trial c9741/cancer and leukemia group b trial 9741. J Clin Oncol 2003;21:1431–9.
- Dale DC. Colony-stimulating factors for the management of neutropenia in cancer patients. Drugs 2002;62(Suppl 1): 1–15.
- Mey UJ, Maier A, Schmidt-Wolf IG, Ziske C, Forstbauer H, Banat GA, et al. Pegfilgrastim as hematopoietic support for dose-dense chemoimmunotherapy with R-CHOP-14 as first-line therapy in elderly patients with diffuse large B cell lymphoma. Support Care Cancer 2007;15:877–84.
- Brusamolino E, Rusconi C, Montalbetti L, Gargantini L, Uziel L, Pinotti G, et al. Dose-dense R-CHOP-14 supported by pegfilgrastim in patients with diffuse large B-cell lymphoma: A phase II study of feasibility and toxicity. Haematologica 2006;91:496–502.
- Tolaney SM, Partridge AH, Sheib RG, Burstein HJ, Winer EP. Pneumocystis carinii pneumonia during dose-dense chemotherapy for breast cancer. J Clin Oncol 2006;24:5330–1.
- Semb KA, Aamdal S, Oian P. Capillary protein leak syndrome appears to explain fluid retention in cancer patients who receive docetaxel treatment. J Clin Oncol 1998; 16:3426–32.
- Piccart MJ, Klijn J, Paridaens R, Nooij M, Mauriac L, Coleman R, et al. Corticosteroids significantly delay the onset of docetaxel-induced fluid retention: Final results of a randomized study of the European Organization for Research and Treatment of Cancer Investigational Drug Branch for Breast Cancer. J Clin Oncol 1997;15: 3149–55.
- Khan A, McNally D, Tutschka PJ, Bilgrami S. Paclitaxel-induced acute bilateral pneumonitis. Ann Pharmacother 1997;31:1471–4.
- Ramanathan RK, Reddy VV, Holbert JM, Belani CP. Pulmonary infiltrates following administration of paclitaxel. Chest 1996;110:289–92.
- Fujimori K, Yokoyama A, Kurita Y, Uno K, Saijo N. Paclitaxel-induced cell-mediated hypersensitivity pneumonitis. Diagnosis using leukocyte migration test, bronchoalveolar lavage and transbronchial lung biopsy. Oncology 1998;55:340–4.
- Goldberg HL, Vannice SB. Pneumonitis related to treatment with paclitaxel. J Clin Oncol 1995;13:534–5.